Viral tracing identifies distributed columnar organization in the olfactory bulb
- PMID: 16895993
- PMCID: PMC1567923
- DOI: 10.1073/pnas.0602032103
Viral tracing identifies distributed columnar organization in the olfactory bulb
Abstract
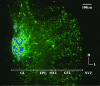
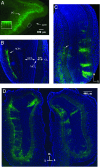
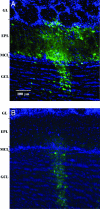
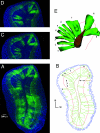
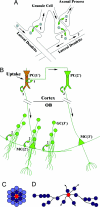
Olfactory sensory neurons converge onto glomeruli in the olfactory bulb (OB) to form modular information processing units. Similar input modules are organized in translaminar columns for other sensory modalities. It has been less clear in the OB whether the initial modular organization relates to a columnar structure in the deeper layers involved in local circuit processing. To probe synaptic connectivity in the OB, we injected a retrograde-specific strain of the pseudorabies virus into the rat OB and piriform cortex. The viral-staining patterns revealed a striking columnar organization that extended across all layers of the OB from the glomeruli to the deep granule cell layer. We hypothesize that the columns represent an extension of the glomerular unit. Specific patterning was observed, suggesting selective, rather than distance-dependent, center-surround connectivity. The results provide a previously undescribed basis for interpreting the synaptic connections between mitral and granule cells within the context of a columnar organization in the OB and have implications for olfactory coding and network organization.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Similar articles
-
Odor exposure reveals non-uniform expression profiles of c-Jun protein in rat olfactory bulb neurons.Brain Res. 1997 Nov 7;774(1-2):142-8. doi: 10.1016/s0006-8993(97)81697-6. Brain Res. 1997. PMID: 9452202
-
Lactosamine differentially affects olfactory sensory neuron projections to the olfactory bulb.Dev Neurobiol. 2007 Oct;67(12):1627-40. doi: 10.1002/dneu.20536. Dev Neurobiol. 2007. PMID: 17567839
-
Examination of morphological and synaptic features of calbindin-immunoreactive neurons in deep layers of the rat olfactory bulb with correlative laser and volume electron microscopy.Microscopy (Oxf). 2019 Aug 6;68(4):316-329. doi: 10.1093/jmicro/dfz019. Microscopy (Oxf). 2019. PMID: 31062844
-
Coding and synaptic processing of sensory information in the glomerular layer of the olfactory bulb.Semin Cell Dev Biol. 2006 Aug;17(4):411-23. doi: 10.1016/j.semcdb.2006.04.007. Epub 2006 May 5. Semin Cell Dev Biol. 2006. PMID: 16765614 Review.
-
Functional organization of the main olfactory bulb.Microsc Res Tech. 1993 Feb 1;24(2):142-56. doi: 10.1002/jemt.1070240206. Microsc Res Tech. 1993. PMID: 8457726 Review.
Cited by
-
Genetic approaches to reveal the connectivity of adult-born neurons.Front Neurosci. 2011 Apr 5;5:48. doi: 10.3389/fnins.2011.00048. eCollection 2011. Front Neurosci. 2011. PMID: 21519388 Free PMC article.
-
Lateral Connectivity in the Olfactory Bulb is Sparse and Segregated.Front Neural Circuits. 2011 Apr 25;5:5. doi: 10.3389/fncir.2011.00005. eCollection 2011. Front Neural Circuits. 2011. PMID: 21559072 Free PMC article.
-
Parallel odor processing by mitral and middle tufted cells in the olfactory bulb.Sci Rep. 2018 May 16;8(1):7625. doi: 10.1038/s41598-018-25740-x. Sci Rep. 2018. PMID: 29769664 Free PMC article.
-
Intraglomerular gap junctions enhance interglomerular synchrony in a sparsely connected olfactory bulb network.J Physiol. 2017 Sep 1;595(17):5965-5986. doi: 10.1113/JP274408. Epub 2017 Jul 23. J Physiol. 2017. PMID: 28640508 Free PMC article.
-
Odor-evoked layer-specific fMRI activities in the awake mouse olfactory bulb.Neuroimage. 2023 Jul 1;274:120121. doi: 10.1016/j.neuroimage.2023.120121. Epub 2023 Apr 18. Neuroimage. 2023. PMID: 37080347 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

