Increased response to morphine in mice lacking protein kinase C epsilon
- PMID: 16899053
- PMCID: PMC4264050
- DOI: 10.1111/j.1601-183X.2006.00261.x
Increased response to morphine in mice lacking protein kinase C epsilon
Abstract
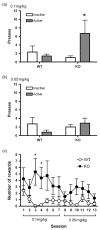
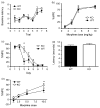
The protein kinase C (PKC) family of serine-threonine kinases has been implicated in behavioral responses to opiates, but little is known about the individual PKC isozymes involved. Here, we show that mice lacking PKCepsilon have increased sensitivity to the rewarding effects of morphine, revealed as the expression of place preference and intravenous self-administration at very low doses of morphine that do not evoke place preference or self-administration in wild-type mice. The PKCepsilon null mice also show prolonged maintenance of morphine place preference in response to repeated testing when compared with wild-type mice. The supraspinal analgesic effects of morphine are enhanced in PKCepsilon null mice, and the development of tolerance to the spinal analgesic effects of morphine is delayed. The density of mu-opioid receptors and their coupling to G-proteins are normal. These studies identify PKCepsilon as a key regulator of opiate sensitivity in mice.
Figures


References
-
- Alessi DR. The protein kinase C inhibitors Ro 318220 and GF 109203X are equally potent inhibitors of MAPKAP kinase-1β (Rsk-2) and p70 S6 kinase. FEBS Lett. 1997;402:121–123. - PubMed
-
- Bailey CP, Connor M. Opioids: cellular mechanisms of tolerance and physical dependence. Curr Opin Pharmacol. 2005;5:60–68. - PubMed
-
- Bailey CP, Kelly E, Henderson G. Protein kinase C activation enhances morphine-induced rapid desensitization of mu-opioid receptors in mature rat locus ceruleus neurons. Mol Pharmacol. 2004;66:1592–1598. - PubMed
-
- Balmberg AB, Bannon AW. Models of nociception: hotplate, tail-flick, and formalin tests in rodents. In: Taylor GP, editor. Current Protocols in Neuroscience. Vol. 2. John Wiley and Sons; Hoboken, NJ: 1999. pp. 8.9.1–8.9.15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

