Developmental and cell-selective variations in N-methyl-D-aspartate receptor degradation by calpain
- PMID: 16899064
- PMCID: PMC2483508
- DOI: 10.1111/j.1471-4159.2006.04096.x
Developmental and cell-selective variations in N-methyl-D-aspartate receptor degradation by calpain
Abstract
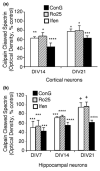
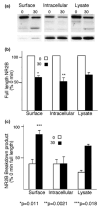
NMDA receptors play critical roles in synaptic modulation and neurological disorders. In this study, we investigated the developmental changes in NR2 cleavage by NMDA receptor-activated calpain in cultured cortical and hippocampal neurons. Calpain activity increased with development, associated with increased expression of NMDA receptors but not of calpain I. The activation of calpain in immature and mature cortical cultures was inhibited by antagonists of NR1/2B and NR1/2A/2B receptors, whereas the inhibition of NR1/2B receptors did not alter calpain activation in mature hippocampal cultures. The degradation of NR2 subunits by calpain differed with developmental age. NR2A was not a substrate of calpain in mature hippocampal cultures, but was cleaved in immature cortical and hippocampal cultures. NR2B degradation by calpain in cortical cultures decreased with development, but the level of degradation of NR2B in hippocampal cultures did not change. The kinetics of NMDA receptor-gated whole cell currents were also modulated by calpain activation in a manner that varied with developmental stage in vitro. In early (but not later) developmental stages, calpain activation altered the NMDA-evoked current rise time and time constants for both desensitization and deactivation. Our data suggest that the susceptibility of the NMDA receptor to cleavage by calpain varies with neuronal maturity in a manner that may alter its electrophysiological properties.
Figures






Similar articles
-
Interactions of postsynaptic density-95 and the NMDA receptor 2 subunit control calpain-mediated cleavage of the NMDA receptor.J Neurosci. 2004 Dec 8;24(49):11035-45. doi: 10.1523/JNEUROSCI.3722-04.2004. J Neurosci. 2004. PMID: 15590920 Free PMC article.
-
Selective activation induced cleavage of the NR2B subunit by calpain.J Neurosci. 2003 Dec 10;23(36):11322-31. doi: 10.1523/JNEUROSCI.23-36-11322.2003. J Neurosci. 2003. PMID: 14672996 Free PMC article.
-
Development and subunit composition of synaptic NMDA receptors in the amygdala: NR2B synapses in the adult central amygdala.J Neurosci. 2003 Jul 30;23(17):6876-83. doi: 10.1523/JNEUROSCI.23-17-06876.2003. J Neurosci. 2003. PMID: 12890782 Free PMC article.
-
Developmental changes in NMDA receptor glycine affinity and ifenprodil sensitivity reveal three distinct populations of NMDA receptors in individual rat cortical neurons.J Neurosci. 1998 Mar 15;18(6):1935-43. doi: 10.1523/JNEUROSCI.18-06-01935.1998. J Neurosci. 1998. PMID: 9482779 Free PMC article. Review.
-
The NR2B subtype of NMDA receptor: a potential target for the treatment of alcohol dependence.Curr Drug Targets CNS Neurol Disord. 2004 Jun;3(3):169-79. doi: 10.2174/1568007043337409. Curr Drug Targets CNS Neurol Disord. 2004. PMID: 15180478 Review.
Cited by
-
The NMDA receptor as a target for cognitive enhancement.Neuropharmacology. 2013 Jan;64:13-26. doi: 10.1016/j.neuropharm.2012.06.051. Epub 2012 Jul 11. Neuropharmacology. 2013. PMID: 22796429 Free PMC article. Review.
-
Distinct regulation of tonic GABAergic inhibition by NMDA receptor subtypes.Cell Rep. 2021 Nov 9;37(6):109960. doi: 10.1016/j.celrep.2021.109960. Cell Rep. 2021. PMID: 34758303 Free PMC article.
-
The Role of GluN2A in Cerebral Ischemia: Promoting Neuron Death and Survival in the Early Stage and Thereafter.Mol Neurobiol. 2018 Feb;55(2):1208-1216. doi: 10.1007/s12035-017-0395-8. Epub 2017 Jan 19. Mol Neurobiol. 2018. PMID: 28102473 Review.
-
Distribution of extrasynaptic NMDA receptors on neurons.ScientificWorldJournal. 2012;2012:267120. doi: 10.1100/2012/267120. Epub 2012 Apr 30. ScientificWorldJournal. 2012. PMID: 22654580 Free PMC article. Review.
-
Calpain 1 and Calpastatin expression is developmentally regulated in rat brain.Exp Neurol. 2009 Dec;220(2):316-9. doi: 10.1016/j.expneurol.2009.09.004. Epub 2009 Sep 12. Exp Neurol. 2009. PMID: 19751724 Free PMC article.
References
-
- Bi R, Bi X, Baudry M. Phosphorylation regulates calpain-mediated truncation of glutamate ionotropic receptors. Brain Res. 1998;797:154–158. - PubMed
-
- Dichter M. Rat cortical neurons in cell culture: culture methods, cell morphology, electrophysiology, and synapse formation. Brain Res. 1978;149:279–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

