Down-regulation of c-Myc following MEK/ERK inhibition halts the expression of malignant phenotype in rhabdomyosarcoma and in non muscle-derived human tumors
- PMID: 16899113
- PMCID: PMC1560159
- DOI: 10.1186/1476-4598-5-31
Down-regulation of c-Myc following MEK/ERK inhibition halts the expression of malignant phenotype in rhabdomyosarcoma and in non muscle-derived human tumors
Abstract
Background: Expression of c-myc proto-oncogene is inappropriate in a wide range of human tumors, and is a downstream target of Ras/Raf/ERK pathway, which promotes c-Myc stability by enhancing c-Myc expression and activity. The aim of this study was to investigate whether the oncogenic phenotype in the human muscle-derived Rhabdomyosarcoma (RD) cell line and in non muscle-derived human tumor cell lines (SW403, IGR39 and PC3) can be blocked by disrupting the c-Myc pathway either by means of pharmacological MEK/ERK inhibition or by direct inactivation of the c-Myc protein.
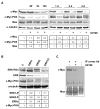
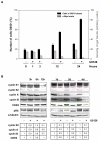
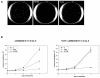
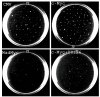
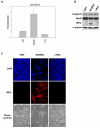
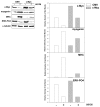
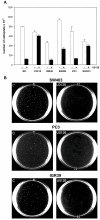
Results: We demonstrate that, in all the tumor cell lines used, the MEK/ERK inhibitor U0126 rapidly induces c-Myc de-phosphorylation, which is followed by a marked reduction in its expression level, by inhibition of proliferation and by reversion of anchorage-independent growth. These data suggest that the targeting of pathways controlling c-Myc expression or stability reverses deregulated growth of different tumor-derived cell lines. Indeed, in RD cells, we found a marked down-regulation of cyclins E2, A and B and CDK2, all of which are known to be targets of c-Myc. Moreover, ectopic MadMyc chimera, a c-Myc function antagonist, causes dramatic growth arrest, CDK and cyclin modulation as well as inhibition of anchorage-independent growth in RD cells, as occurs in U0126-treated cells. In particular, we found that the mere inhibition of c-Myc by MadMyc chimera rescues the myogenic program, MHC expression and the acquisition of the myogenic-like phenotype in RD cells.
Conclusion: Our data provide evidence of the key role played by the MEK/ERK pathway in the growth arrest and transformation phenotype of Rhabdomyosarcoma and of non muscle-derived tumor cell lines. In fact, MEK/ERK inhibitor, U0126, induces growth arrest, anchorage-dependent growth of these cell lines. In addition, the results of this study demonstrate that the direct inactivation of c-Myc by Mad/Myc chimera rescues myogenic program and leads to the reversal of the Rhabdomyosarcoma phenotype. In conclusion these data strongly suggest that the targeting of c-Myc by means of the MEK inhibitor can be tested as a promising strategy in anti-cancer therapy.
Figures

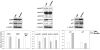


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

