Mutant analysis of the Shal (Kv4) voltage-gated fast transient K+ channel in Caenorhabditis elegans
- PMID: 16899454
- PMCID: PMC2259281
- DOI: 10.1074/jbc.M605814200
Mutant analysis of the Shal (Kv4) voltage-gated fast transient K+ channel in Caenorhabditis elegans
Abstract
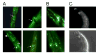
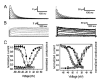
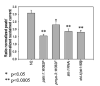
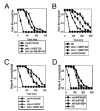
Shal (Kv4) alpha-subunits are the most conserved among the family of voltage-gated potassium channels. Previous work has shown that the Shal potassium channel subfamily underlies the predominant fast transient outward current in Drosophila neurons (Tsunoda, S., and Salkoff, L. (1995) J. Neurosci. 15, 1741-1754) and the fast transient outward current in mouse heart muscle (Guo, W., Jung, W. E., Marionneau, C., Aimond, F., Xu, H., Yamada, K. A., Schwarz, T. L., Demolombe, S., and Nerbonne, J. M. (2005) Circ. Res. 97, 1342-1350). We show that Shal channels also play a role as the predominant transient outward current in Caenorhabditis elegans muscle. Green fluorescent protein promoter experiments also revealed SHL-1 expression in a subset of neurons as well as in C. elegans body wall muscle and in male-specific diagonal muscles. The shl-1 (ok1168) null mutant removed all fast transient outward current from muscle cells. SHL-1 currents strongly resembled Shal currents in other species except that they were active in a more depolarized voltage range. We also determined that the remaining delayed-rectifier current in cultured myocytes was carried by the Shaker ortholog SHK-1. In shl-1 (ok1168) mutants there was a significant compensatory increase in the SHK-1 current. Male shl-1 (ok1168) animals exhibited reduced mating efficiency resulting from an apparent difficulty in locating the hermaphrodite vulva. SHL-1 channels are apparently important in fine-tuning complex behaviors, such as mating, that play a crucial role in the survival and propagation of the species.
Figures



Similar articles
-
KChIP-like auxiliary subunits of Kv4 channels regulate excitability of muscle cells and control male turning behavior during mating in Caenorhabditis elegans.J Neurosci. 2015 Feb 4;35(5):1880-91. doi: 10.1523/JNEUROSCI.3429-14.2015. J Neurosci. 2015. PMID: 25653349 Free PMC article.
-
Shal and shaker differential contribution to the K+ currents in the Drosophila mushroom body neurons.J Neurosci. 2005 Mar 2;25(9):2348-58. doi: 10.1523/JNEUROSCI.4384-04.2005. J Neurosci. 2005. PMID: 15745961 Free PMC article.
-
Dissection of K+ currents in Caenorhabditis elegans muscle cells by genetics and RNA interference.Proc Natl Acad Sci U S A. 2003 Nov 25;100(24):14391-6. doi: 10.1073/pnas.1935976100. Epub 2003 Nov 11. Proc Natl Acad Sci U S A. 2003. PMID: 14612577 Free PMC article.
-
Pharmacological Approaches for the Modulation of the Potassium Channel KV4.x and KChIPs.Int J Mol Sci. 2021 Jan 31;22(3):1419. doi: 10.3390/ijms22031419. Int J Mol Sci. 2021. PMID: 33572566 Free PMC article. Review.
-
Shal-type (Kv4.x) potassium channel pore blockers from scorpion venoms.Sheng Li Xue Bao. 2015 Jun 25;67(3):248-54. Sheng Li Xue Bao. 2015. PMID: 26109297 Review.
Cited by
-
CLHM-1 is a functionally conserved and conditionally toxic Ca2+-permeable ion channel in Caenorhabditis elegans.J Neurosci. 2013 Jul 24;33(30):12275-86. doi: 10.1523/JNEUROSCI.5919-12.2013. J Neurosci. 2013. PMID: 23884934 Free PMC article.
-
Motor Rhythm Dissection From the Backward Circuit in C. elegans.Front Mol Neurosci. 2022 Mar 16;15:845733. doi: 10.3389/fnmol.2022.845733. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35370545 Free PMC article.
-
C. elegans enteric motor neurons fire synchronized action potentials underlying the defecation motor program.Nat Commun. 2022 May 19;13(1):2783. doi: 10.1038/s41467-022-30452-y. Nat Commun. 2022. PMID: 35589790 Free PMC article.
-
Cell-specific proteomic analysis in Caenorhabditis elegans.Proc Natl Acad Sci U S A. 2015 Mar 3;112(9):2705-10. doi: 10.1073/pnas.1421567112. Epub 2015 Feb 17. Proc Natl Acad Sci U S A. 2015. PMID: 25691744 Free PMC article.
-
Serotonin activates overall feeding by activating two separate neural pathways in Caenorhabditis elegans.J Neurosci. 2012 Feb 8;32(6):1920-31. doi: 10.1523/JNEUROSCI.2064-11.2012. J Neurosci. 2012. PMID: 22323705 Free PMC article.
References
-
- Guo W, Jung WE, Marionneau C, Aimond F, Xu H, Yamada KA, Schwarz TL, Demolombe S, Nerbonne JM. Circ Res. 2005;97:1342–1350. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

