Unique biologic properties of recombinant AAV1 transduction in polarized human airway epithelia
- PMID: 16899463
- PMCID: PMC1712671
- DOI: 10.1074/jbc.M604099200
Unique biologic properties of recombinant AAV1 transduction in polarized human airway epithelia
Abstract
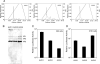
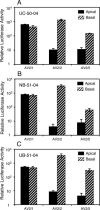
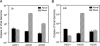
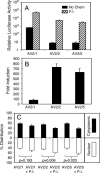
The choice of adeno-associated virus serotypes for clinical applications is influenced by the animal model and model system used to evaluate various serotypes. In the present study, we sought to compare the biologic properties of rAAV2/1, rAAV2/2, and rAAV2/5 transduction in polarized human airway epithelia using viruses purified by a newly developed common column chromatography method. Results demonstrated that apical transduction of human airway epithelia with rAAV2/1 was 100-fold more efficient than rAAV2/2 and rAAV2/5. This transduction profile in human airway epithelia (rAAV2/1 >> rAAV2/2 = rAAV2/5) was significantly different from that seen following nasal administration of these vectors to mouse lung (rAAV2/5 > rAAV2/1 >> rAAV2/2), emphasizing differences in transduction of these serotypes between these two species. In stark contrast to rAAV2/2 and rAAV2/5, rAAV2/1 transduced both the apical and basolateral membrane of human airway epithelia with similar efficiency. However, the overall level of transduction across serotypes did not correlate with vector internalization. We hypothesized that differences in post-entry processing of these serotypes might influence the efficiency of apical transduction. To this end, we tested the effectiveness of proteasome inhibitors to augment nuclear translocation and gene expression from the three serotypes. Augmentation of rAAV2/1 apical transduction of human polarized airway epithelia was 10-fold lower than that for rAAV2/2 and rAAV2/5. Cellular fractionation studies demonstrated that proteasome inhibitors more significantly enhanced rAAV2/2 and rAAV2/5 translocation to the nucleus than rAAV2/1. These results demonstrate that AAV1 transduction biology in human airway epithelia differs from that of AAV2 and AAV5 by virtue of altered ubiquitin/proteasome sensitivities that influence nuclear translocation.
Figures

References
-
- Blacklow NR. In: Parvoviruses and Human Disease. Pattison JR, editor. CRC Press; Boca Raton, FL: 1988. pp. 165–174.
-
- Grimm D, Kay MA. Curr. Gene. Ther. 2003;3:281–304. - PubMed
-
- Mori S, Wang L, Takeuchi T, Kanda T. Virology. 2004;330:375–383. - PubMed
-
- Gao G, Vandenberghe LH, Wilson JM. Curr. Gene. Ther. 2005;5:285–297. - PubMed
-
- Louboutin JP, Wang L, Wilson JM. J. Gene. Med. 2005;7:442–451. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

