Inhibition of protein aggregation in vitro and in vivo by a natural osmoprotectant
- PMID: 16899544
- PMCID: PMC1569168
- DOI: 10.1073/pnas.0603772103
Inhibition of protein aggregation in vitro and in vivo by a natural osmoprotectant
Abstract
Small organic molecules termed osmolytes are harnessed by a variety of cell types in a wide range of organisms to counter unfavorable physiological conditions that challenge protein stability and function. Using a well characterized reporter system that we developed to allow in vivo observations, we have explored how the osmolyte proline influences the stability and aggregation of a model aggregation-prone protein, P39A cellular retinoic acid-binding protein. Strikingly, we find that the natural osmolyte proline abrogates aggregation both in vitro and in vivo (in an Escherichia coli expression system). Importantly, proline also prevented aggregation of constructs containing exon 1 of huntingtin with extended polyglutamine tracts. Although compatible osmolytes are known to stabilize the native state, our results point to a destabilizing effect of proline on partially folded states and early aggregates and a solubilizing effect on the native state. Because proline is believed to act through a combination of solvophobic backbone interactions and favorable side-chain interactions that are not specific to a particular sequence or structure, the observed effect is likely to be general. Thus, the osmolyte proline may be protective against biomedically important protein aggregates that are hallmarks of several late-onset neurodegenerative diseases including Huntington's, Alzheimer's, and Parkinson's. In addition, these results should be of practical importance because they may enable protein expression at higher efficiency under conditions where aggregation competes with proper folding.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
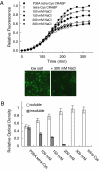
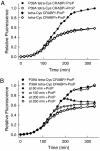
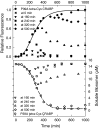
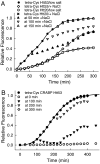
Comment in
-
Proline to the rescue.Proc Natl Acad Sci U S A. 2006 Sep 5;103(36):13265-6. doi: 10.1073/pnas.0606106103. Epub 2006 Aug 28. Proc Natl Acad Sci U S A. 2006. PMID: 16938858 Free PMC article. No abstract available.
Similar articles
-
Proline to the rescue.Proc Natl Acad Sci U S A. 2006 Sep 5;103(36):13265-6. doi: 10.1073/pnas.0606106103. Epub 2006 Aug 28. Proc Natl Acad Sci U S A. 2006. PMID: 16938858 Free PMC article. No abstract available.
-
Aggregation of a slow-folding mutant of a beta-clam protein proceeds through a monomeric nucleus.Biochemistry. 2005 May 17;44(19):7266-74. doi: 10.1021/bi047404e. Biochemistry. 2005. PMID: 15882065
-
In-cell aggregation of a polyglutamine-containing chimera is a multistep process initiated by the flanking sequence.J Biol Chem. 2007 Dec 14;282(50):36736-43. doi: 10.1074/jbc.M703682200. Epub 2007 Oct 17. J Biol Chem. 2007. PMID: 17942400 Free PMC article.
-
The osmophobic effect: natural selection of a thermodynamic force in protein folding.J Mol Biol. 2001 Jul 27;310(5):955-63. doi: 10.1006/jmbi.2001.4819. J Mol Biol. 2001. PMID: 11502004 Review.
-
Peptide aggregation in neurodegenerative disease.Annu Rev Biomed Eng. 2002;4:155-74. doi: 10.1146/annurev.bioeng.4.092801.094202. Epub 2002 Mar 22. Annu Rev Biomed Eng. 2002. PMID: 12117755 Review.
Cited by
-
Sigma Factor Engineering in Actinoplanes sp. SE50/110: Expression of the Alternative Sigma Factor Gene ACSP50_0507 (σHAs) Enhances Acarbose Yield and Alters Cell Morphology.Microorganisms. 2024 Jun 20;12(6):1241. doi: 10.3390/microorganisms12061241. Microorganisms. 2024. PMID: 38930623 Free PMC article.
-
Osmoprotection of Bacillus subtilis through import and proteolysis of proline-containing peptides.Appl Environ Microbiol. 2013 Jan;79(2):576-87. doi: 10.1128/AEM.01934-12. Epub 2012 Nov 9. Appl Environ Microbiol. 2013. PMID: 23144141 Free PMC article.
-
Functional polymorphisms in PRODH are associated with risk and protection for schizophrenia and fronto-striatal structure and function.PLoS Genet. 2008 Nov;4(11):e1000252. doi: 10.1371/journal.pgen.1000252. Epub 2008 Nov 7. PLoS Genet. 2008. PMID: 18989458 Free PMC article.
-
The green tea polyphenol (-)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios.FEBS Lett. 2015 Jan 2;589(1):77-83. doi: 10.1016/j.febslet.2014.11.026. Epub 2014 Nov 29. FEBS Lett. 2015. PMID: 25436420 Free PMC article.
-
The Multifaceted Roles of Proline in Cell Behavior.Front Cell Dev Biol. 2021 Aug 12;9:728576. doi: 10.3389/fcell.2021.728576. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34458276 Free PMC article. Review.
References
-
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Science. 1982;217:1214–1222. - PubMed
-
- Yancey P. H. J. Exp. Biol. 2005;208:2819–2830. - PubMed
-
- Record M. T., Jr., Courtenay E. S., Cayley D. S., Guttman H. J. Trends Biochem. Sci. 1998;23:143–148. - PubMed
-
- Bolen D. W., Baskakov I. V. J. Mol. Biol. 2001;310:955–963. - PubMed
-
- Cayley S., Record M. T., Jr. Biochemistry. 2003;42:12596–12609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

