Cellular mechanisms underlying prostaglandin-induced transient cAMP signals near the plasma membrane of HEK-293 cells
- PMID: 16899551
- PMCID: PMC4712347
- DOI: 10.1152/ajpcell.00121.2006
Cellular mechanisms underlying prostaglandin-induced transient cAMP signals near the plasma membrane of HEK-293 cells
Abstract
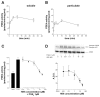
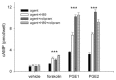
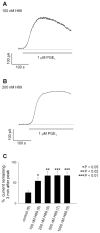
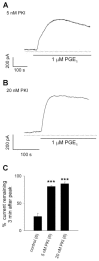
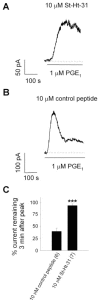
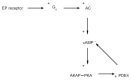
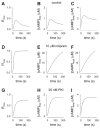
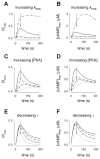
We have previously used cyclic nucleotide-gated (CNG) channels as sensors to measure cAMP signals in human embryonic kidney (HEK)-293 cells. We found that prostaglandin E(1) (PGE(1)) triggered transient increases in cAMP concentration near the plasma membrane, whereas total cAMP levels rose to a steady plateau over the same time course. In addition, we presented evidence that the decline in the near-membrane cAMP levels was due primarily to a PGE(1)-induced stimulation of phosphodiesterase (PDE) activity, and that the differences between near-membrane and total cAMP levels were largely due to diffusional barriers and differential PDE activity. Here, we examine the mechanisms regulating transient, near-membrane cAMP signals. We observed that 5-min stimulation of HEK-293 cells with prostaglandins triggered a two- to threefold increase in PDE4 activity. Extracellular application of H89 (a PKA inhibitor) inhibited stimulation of PDE4 activity. Similarly, when we used CNG channels to monitor cAMP signals we found that both extracellular and intracellular (via the whole-cell patch pipette) application of H89, or the highly selective PKA inhibitor, PKI, prevented the decline in prostaglandin-induced responses. Following pretreatment with rolipram (a PDE4 inhibitor), H89 had little or no effect on near-membrane or total cAMP levels. Furthermore, disrupting the subcellular localization of PKA with the A-kinase anchoring protein (AKAP) disruptor Ht31 prevented the decline in the transient response. Based on these data we developed a plausible kinetic model that describes prostaglandin-induced cAMP signals. This model has allowed us to quantitatively demonstrate the importance of PKA-mediated stimulation of PDE4 activity in shaping near-membrane cAMP signals.
Figures


Similar articles
-
Roles of GRK and PDE4 activities in the regulation of beta2 adrenergic signaling.J Gen Physiol. 2008 Apr;131(4):349-64. doi: 10.1085/jgp.200709881. Epub 2008 Mar 17. J Gen Physiol. 2008. PMID: 18347080 Free PMC article.
-
Negative feedback exerted by cAMP-dependent protein kinase and cAMP phosphodiesterase on subsarcolemmal cAMP signals in intact cardiac myocytes: an in vivo study using adenovirus-mediated expression of CNG channels.J Biol Chem. 2004 Dec 10;279(50):52095-105. doi: 10.1074/jbc.M405697200. Epub 2004 Oct 1. J Biol Chem. 2004. PMID: 15466415
-
beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi.Proc Natl Acad Sci U S A. 2003 Feb 4;100(3):940-5. doi: 10.1073/pnas.262787199. Epub 2003 Jan 27. Proc Natl Acad Sci U S A. 2003. Retraction in: Proc Natl Acad Sci U S A. 2022 Apr 26;119(17):e2205198119. doi: 10.1073/pnas.2205198119. PMID: 12552097 Free PMC article. Retracted.
-
The mAKAP signaling complex: integration of cAMP, calcium, and MAP kinase signaling pathways.Eur J Cell Biol. 2006 Jul;85(7):593-602. doi: 10.1016/j.ejcb.2006.01.007. Epub 2006 Feb 7. Eur J Cell Biol. 2006. PMID: 16460834 Review.
-
Arrestin times for compartmentalised cAMP signalling and phosphodiesterase-4 enzymes.Curr Opin Cell Biol. 2005 Apr;17(2):129-34. doi: 10.1016/j.ceb.2005.01.003. Curr Opin Cell Biol. 2005. PMID: 15780588 Review.
Cited by
-
Cyclic nucleotide phosphodiesterase 1A: a key regulator of cardiac fibroblast activation and extracellular matrix remodeling in the heart.Basic Res Cardiol. 2011 Nov;106(6):1023-39. doi: 10.1007/s00395-011-0228-2. Epub 2011 Oct 20. Basic Res Cardiol. 2011. PMID: 22012077 Free PMC article.
-
Cyclic AMP compartments and signaling specificity: role of cyclic nucleotide phosphodiesterases.J Gen Physiol. 2014 Jan;143(1):29-38. doi: 10.1085/jgp.201311083. J Gen Physiol. 2014. PMID: 24378905 Free PMC article. No abstract available.
-
Assessment of cellular mechanisms contributing to cAMP compartmentalization in pulmonary microvascular endothelial cells.Am J Physiol Cell Physiol. 2012 Mar 15;302(6):C839-52. doi: 10.1152/ajpcell.00361.2011. Epub 2011 Nov 23. Am J Physiol Cell Physiol. 2012. PMID: 22116306 Free PMC article.
-
A2B adenosine receptors inhibit superoxide production from mitochondrial complex I in rabbit cardiomyocytes via a mechanism sensitive to Pertussis toxin.Br J Pharmacol. 2011 Jul;163(5):995-1006. doi: 10.1111/j.1476-5381.2011.01288.x. Br J Pharmacol. 2011. PMID: 21366548 Free PMC article.
-
Cyclic AMP phosphodiesterase 4D (PDE4D) Tethers EPAC1 in a vascular endothelial cadherin (VE-Cad)-based signaling complex and controls cAMP-mediated vascular permeability.J Biol Chem. 2010 Oct 29;285(44):33614-22. doi: 10.1074/jbc.M110.140004. Epub 2010 Aug 23. J Biol Chem. 2010. PMID: 20732872 Free PMC article.
References
-
- Barnes AP, Livera G, Huang P, Sun C, O’Neal WK, Conti M, Stutts MJ, Milgram SL. Phosphodiesterase 4D forms a cAMP diffusion barrier at the apical membrane of the airway epithelium. J Biol Chem. 2005;280:7997–8003. - PubMed
-
- Brunton LL, Hayes JS, Mayer SE. Functional compartmentation of cAMP and protein kinase in heart. Adv Cyclic Nucleotide Res. 1981;14:391–397. - PubMed
-
- Carr DW, Stofko-Hahn RE, Fraser ID, Bishop SM, Acott TS, Brennan RG, Scott JD. Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif. J Biol Chem. 1991;266:14188–14192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

