Effects of sensing behavior on a latency code
- PMID: 16899717
- PMCID: PMC6673807
- DOI: 10.1523/JNEUROSCI.1508-06.2006
Effects of sensing behavior on a latency code
Abstract
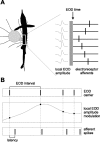
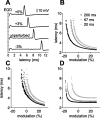
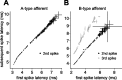
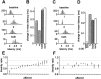
Sensory information is often acquired through active exploration, yet relatively little is known about how neurons encode sensory stimuli in the context of natural patterns of sensing behavior. We examined the effects of sensing behavior on a spike latency code in the active electrosensory system of mormyrid fish. These fish actively probe their environment by emitting brief electric organ discharge (EOD) pulses. Nearby objects alter the spatial pattern of current flowing through the skin. These changes are encoded by small shifts in the latency of individual electroreceptor afferent spikes after the EOD. In nature, the temporal pattern of EOD intervals is highly structured and varies depending on the behavioral context. We performed experiments in which we varied both the EOD amplitude and the intervals between EODs to understand how sensing behavior affects afferent latency coding. We use white-noise stimuli and linear filter estimation methods to develop simple models characterizing the dependence of afferent spike latency on the preceding sequence of EOD intervals and amplitudes. Comparing the predictions of these models with actual afferent responses for natural patterns of EOD intervals and amplitudes reveals an unexpectedly rich interplay between sensing behavior and stimulus encoding. Implications of our results for how afferent spike latency is decoded at central stages of electrosensory processing are discussed.
Figures




Similar articles
-
Signal Diversification Is Associated with Corollary Discharge Evolution in Weakly Electric Fish.J Neurosci. 2020 Aug 12;40(33):6345-6356. doi: 10.1523/JNEUROSCI.0875-20.2020. Epub 2020 Jul 13. J Neurosci. 2020. PMID: 32661026 Free PMC article.
-
Modeling latency code processing in the electric sense: from the biological template to its VLSI implementation.Bioinspir Biomim. 2016 Sep 13;11(5):055007. doi: 10.1088/1748-3190/11/5/055007. Bioinspir Biomim. 2016. PMID: 27623047
-
Sex recognition and neuronal coding of electric organ discharge waveform in the pulse-type weakly electric fish, Hypopomus occidentalis.J Comp Physiol A. 1988 Aug;163(4):465-78. doi: 10.1007/BF00604901. J Comp Physiol A. 1988. PMID: 3184009
-
Electric signaling behavior and the mechanisms of electric organ discharge production in mormyrid fish.J Physiol Paris. 2002 Sep-Dec;96(5-6):405-19. doi: 10.1016/S0928-4257(03)00019-6. J Physiol Paris. 2002. PMID: 14692489 Review.
-
Plasticity of feedback inputs in the apteronotid electrosensory system.J Exp Biol. 1999 May;202(Pt 10):1327-37. doi: 10.1242/jeb.202.10.1327. J Exp Biol. 1999. PMID: 10210673 Review.
Cited by
-
Throwing a glance at the neural code: rapid information transmission in the visual system.HFSP J. 2009;3(1):36-46. doi: 10.2976/1.3027089. Epub 2008 Dec 3. HFSP J. 2009. PMID: 19649155 Free PMC article.
-
An end-to-end model of active electrosensation.Curr Biol. 2025 May 19;35(10):2295-2306.e4. doi: 10.1016/j.cub.2025.03.074. Epub 2025 Apr 23. Curr Biol. 2025. PMID: 40273914
-
Ongoing temporal coding of a stochastic stimulus as a function of intensity: time-intensity trading.J Neurosci. 2012 Jul 11;32(28):9517-27. doi: 10.1523/JNEUROSCI.0103-12.2012. J Neurosci. 2012. PMID: 22787037 Free PMC article.
-
Motor patterns during active electrosensory acquisition.Front Behav Neurosci. 2014 May 28;8:186. doi: 10.3389/fnbeh.2014.00186. eCollection 2014. Front Behav Neurosci. 2014. PMID: 24904337 Free PMC article.
-
Neural readout of a latency code in the active electrosensory system.Cell Rep. 2022 Mar 29;38(13):110605. doi: 10.1016/j.celrep.2022.110605. Cell Rep. 2022. PMID: 35354029 Free PMC article.
References
-
- Attneave F (1954). Some informational aspects of visual perception. Psychol Rev 61:183–193. - PubMed
-
- Barlow H (1961). Possible principles underlying the transformation of sensory messages. In: In: Sensory communication (Rosenblith W, ed.) pp. 217–234. Cambridge, MA: MIT.
-
- Bell CC (1990a). Mormyromast electroreceptor organs and their afferents in mormyrid electric fish. II. Intra-axonal recordings show initial stages of central processing. J Neurophysiol 63:303–318. - PubMed
-
- Bell CC (1990b). Mormyromast electroreceptor organs and their afferents in mormyrid electric fish. III. Physiological differences between two morphological types of fibers. J Neurophysiol 63:319–332. - PubMed
-
- Bell CC, Russell CJ (1978). Termination of electroreceptor and mechanical lateral line afferents in the mormyrid acousticolateral area. J Comp Neurol 182:367–382. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
