Compensation of inositol 1,4,5-trisphosphate receptor function by altering sarco-endoplasmic reticulum calcium ATPase activity in the Drosophila flight circuit
- PMID: 16899722
- PMCID: PMC6673814
- DOI: 10.1523/JNEUROSCI.1231-06.2006
Compensation of inositol 1,4,5-trisphosphate receptor function by altering sarco-endoplasmic reticulum calcium ATPase activity in the Drosophila flight circuit
Abstract
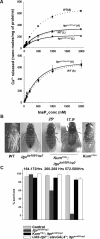
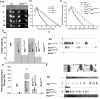
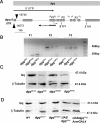
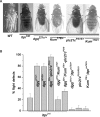
Ionic Ca2+ functions as a second messenger to control several intracellular processes. It also influences intercellular communication. The release of Ca2+ from intracellular stores through the inositol 1,4,5-trisphosphate receptor (InsP3R) occurs in both excitable and nonexcitable cells. In Drosophila, InsP3R activity is required in aminergic interneurons during pupal development for normal flight behavior. By altering intracellular Ca2+ and InsP3 levels through genetic means, we now show that signaling through the InsP3R is required at multiple steps for generating the neural circuit required in air puff-stimulated Drosophila flight. Decreased Ca2+ release in aminergic neurons during development of the flight circuit can be compensated by reducing Ca2+ uptake from the cytosol to intracellular stores. However, this mode of increasing intracellular Ca2+ is insufficient for maintenance of flight patterns over time periods necessary for normal flight. Our study suggests that processes such as maintenance of wing posture and formation of the flight circuit require InsP3 receptor function at a slow timescale and can thus be modulated by altering levels of cytosolic Ca2+ and InsP3. In contrast, maintenance of flight patterns probably requires fast modulation of Ca2+ levels, in which the intrinsic properties of the InsP3R play a pivotal role.
Figures

References
-
- Ashburner M (1989). In: Drosophila, a laboratory handbook Cold Spring Harbor, NY: Cold Spring Harbor Laboratory.
-
- Benzer S (1973). Genetic dissection of behavior. Sci Am 229:24–37. - PubMed
-
- Berridge MJ (1993). Inositol trisphosphate and calcium signalling. Nature 361:315–325. - PubMed
-
- Berridge MJ (1998). Neuronal calcium signaling. Neuron 21:13–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
