Integration of visuospatial and effector information during symbolically cued limb movements in monkey lateral intraparietal area
- PMID: 16899726
- PMCID: PMC6673800
- DOI: 10.1523/JNEUROSCI.1779-06.2006
Integration of visuospatial and effector information during symbolically cued limb movements in monkey lateral intraparietal area
Abstract
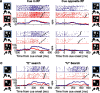
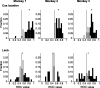
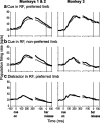
Natural behavior requires close but flexible coordination between attention, defined as selection for perception, and action. In recent years a distributed network including the lateral intraparietal area (LIP) has been implicated in visuospatial selection for attention and rapid eye movements (saccades), but the relation between the attentional and motor functions of this area remains unclear. Here we tested LIP neurons in a task that involved not an ocular but a manual operant response. Monkeys viewed a display containing one cue and several distractors and reported the orientation of the cue (right- or left-facing) by releasing one of two bars grasped, respectively, with the right or left hand. The movement in this task thus was associated with (cued by), but not directed toward, the visual stimulus. A large majority of neurons responded more when the cue rather than when a distractor was in their receptive field, suggesting that they contribute to the attentional selection of the cue. A fraction of these neurons also was modulated by limb release, thus simultaneously encoding cue location and the active limb. The results suggest that the LIP links behaviorally relevant visual information with motor variables relevant for solving a task in a wide range of circumstances involving goal-directed or symbolically cued movements and eye as well as limb movements. A central function of the LIP may be to coordinate visual and motor selection during a wide variety of behaviors.
Figures





References
-
- Barash S, Bracewell RM, Fogassi L, Gnadt JW, Andersen RA (1991). Saccade-related activity in the lateral intraparietal area. I. Temporal properties. J Neurophysiol 66:1095–1108. - PubMed
-
- Bisley JW, Goldberg ME (2003). Neuronal activity in the lateral intraparietal area and spatial attention. Science 299:81–86. - PubMed
-
- Calton JL, Dickinson AR, Snyder LH (2002). Non-spatial, motor-specific activation in posterior parietal cortex. Nat Neurosci 5:580–588. - PubMed
-
- Colby CL, Duhamel J-R, Goldberg ME (1996). Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol 76:2841–2852. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
