Activity bidirectionally regulates AMPA receptor mRNA abundance in dendrites of hippocampal neurons
- PMID: 16899729
- PMCID: PMC6673809
- DOI: 10.1523/JNEUROSCI.0472-06.2006
Activity bidirectionally regulates AMPA receptor mRNA abundance in dendrites of hippocampal neurons
Abstract
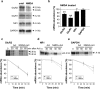
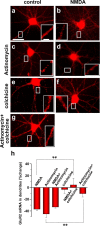
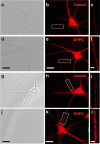
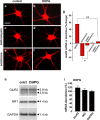
Activity-dependent regulation of synaptic AMPA receptor (AMPAR) number is critical to NMDA receptor (NMDAR)-dependent synaptic plasticity. Using quantitative high-resolution in situ hybridization, we show that mRNAs encoding the AMPA-type glutamate receptor subunits (GluRs) 1 and 2 are localized to dendrites of hippocampal neurons and are regulated by paradigms that alter synaptic efficacy. A substantial fraction of synaptic sites contain AMPAR mRNA, consistent with strategic positioning and availability for "on-site" protein synthesis. NMDAR activation depletes dendritic levels of AMPAR mRNAs. The decrease in mRNA occurs via rise in intracellular Ca2+, activation of extracellular signal-regulated kinase/mitogen-activated protein kinase signaling, and transcriptional arrest at the level of the nucleus. The decrease in mRNA is accompanied by a long-lasting reduction in synaptic AMPAR number, consistent with reduced synaptic efficacy. In contrast, group I metabotropic GluR signaling promotes microtubule-based trafficking of existing AMPAR mRNAs from the soma to dendrites. Bidirectional regulation of dendritic mRNA abundance represents a potentially powerful means to effect long-lasting changes in synaptic strength.
Figures
Similar articles
-
Palmitoylation of A-kinase anchoring protein 79/150 regulates dendritic endosomal targeting and synaptic plasticity mechanisms.J Neurosci. 2012 May 23;32(21):7119-36. doi: 10.1523/JNEUROSCI.0784-12.2012. J Neurosci. 2012. PMID: 22623657 Free PMC article.
-
Modulation of dendritic AMPA receptor mRNA trafficking by RNA splicing and editing.Nucleic Acids Res. 2013 Jan 7;41(1):617-31. doi: 10.1093/nar/gks1223. Epub 2012 Nov 19. Nucleic Acids Res. 2013. PMID: 23166306 Free PMC article.
-
NMDA receptors mediate calcium-dependent, bidirectional changes in dendritic PICK1 clustering.Mol Cell Neurosci. 2006 Mar;31(3):574-85. doi: 10.1016/j.mcn.2005.11.011. Epub 2006 Jan 10. Mol Cell Neurosci. 2006. PMID: 16406232
-
Posttranslational modifications and receptor-associated proteins in AMPA receptor trafficking and synaptic plasticity.Neurosignals. 2006-2007;15(5):266-82. doi: 10.1159/000105517. Epub 2007 Jul 9. Neurosignals. 2006. PMID: 17622793 Review.
-
Role of AMPA receptors in synaptic plasticity.Cell Tissue Res. 2006 Nov;326(2):447-55. doi: 10.1007/s00441-006-0275-4. Epub 2006 Aug 1. Cell Tissue Res. 2006. PMID: 16896950 Review.
Cited by
-
Neuron-wide RNA transport combines with netrin-mediated local translation to spatially regulate the synaptic proteome.Elife. 2015 Jan 8;4:e04158. doi: 10.7554/eLife.04158. Elife. 2015. PMID: 25569157 Free PMC article.
-
Identification of process-localized mRNAs from cultured rodent hippocampal neurons.J Neurosci. 2006 Dec 20;26(51):13390-9. doi: 10.1523/JNEUROSCI.3432-06.2006. J Neurosci. 2006. PMID: 17182790 Free PMC article.
-
Nonspecific interaction of prefibrillar amyloid aggregates with glutamatergic receptors results in Ca2+ increase in primary neuronal cells.J Biol Chem. 2008 Oct 31;283(44):29950-60. doi: 10.1074/jbc.M803992200. Epub 2008 Aug 1. J Biol Chem. 2008. PMID: 18676369 Free PMC article.
-
Activity dependent LoNA regulates translation by coordinating rRNA transcription and methylation.Nat Commun. 2018 Apr 30;9(1):1726. doi: 10.1038/s41467-018-04072-4. Nat Commun. 2018. PMID: 29712923 Free PMC article.
-
GluA2 mRNA distribution and regulation by miR-124 in hippocampal neurons.Mol Cell Neurosci. 2014 Jul;61:1-12. doi: 10.1016/j.mcn.2014.04.006. Epub 2014 Apr 28. Mol Cell Neurosci. 2014. PMID: 24784359 Free PMC article.
References
-
- Allen MP, Xu M, Zeng C, Tobet SA, Wierman ME (2000). Myocyte enhancer factors-2B and -2C are required for adhesion related kinase repression of neuronal gonadotropin releasing hormone gene expression. J Biol Chem 275:39662–39670. - PubMed
-
- Aronov S, Marx R, Ginzburg I (1999). Identification of 3′UTR region implicated in tau mRNA stabilization in neuronal cells. J Mol Neurosci 12:131–145. - PubMed
-
- Barry MF, Ziff EB (2002). Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol 12:279–286. - PubMed
-
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC (2000). Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci 3:1291–1300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
