The role of the ventromedial prefrontal cortex in abstract state-based inference during decision making in humans
- PMID: 16899731
- PMCID: PMC6673813
- DOI: 10.1523/JNEUROSCI.1010-06.2006
The role of the ventromedial prefrontal cortex in abstract state-based inference during decision making in humans
Abstract
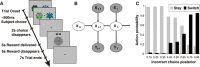
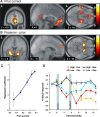
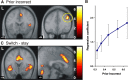
Many real-life decision-making problems incorporate higher-order structure, involving interdependencies between different stimuli, actions, and subsequent rewards. It is not known whether brain regions implicated in decision making, such as the ventromedial prefrontal cortex (vmPFC), use a stored model of the task structure to guide choice (model-based decision making) or merely learn action or state values without assuming higher-order structure as in standard reinforcement learning. To discriminate between these possibilities, we scanned human subjects with functional magnetic resonance imaging while they performed a simple decision-making task with higher-order structure, probabilistic reversal learning. We found that neural activity in a key decision-making region, the vmPFC, was more consistent with a computational model that exploits higher-order structure than with simple reinforcement learning. These results suggest that brain regions, such as the vmPFC, use an abstract model of task structure to guide behavioral choice, computations that may underlie the human capacity for complex social interactions and abstract strategizing.
Figures

Comment in
-
Simple reinforcement learning models are not always appropriate.J Neurosci. 2006 Nov 8;26(45):11511-2. doi: 10.1523/jneurosci.3973-06.2006. J Neurosci. 2006. PMID: 17106946 Free PMC article. Review. No abstract available.
References
-
- Bechara A, Tranel D, Damasio H (2000). Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 123:2189–2202. - PubMed
-
- Camerer CF (2003). Strategizing in the brain. Science 300:1673–1675. - PubMed
-
- Daw ND, Niv Y, Dayan P (2005). Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci 8:1704–1711. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
