Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition
- PMID: 16899778
- PMCID: PMC4178954
- DOI: 10.1056/NEJMoa055137
Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition
Abstract
Background: Neonatal-onset multisystem inflammatory disease is characterized by fever, urticarial rash, aseptic meningitis, deforming arthropathy, hearing loss, and mental retardation. Many patients have mutations in the cold-induced autoinflammatory syndrome 1 (CIAS1) gene, encoding cryopyrin, a protein that regulates inflammation.
Methods: We selected 18 patients with neonatal-onset multisystem inflammatory disease (12 with identifiable CIAS1 mutations) to receive anakinra, an interleukin-1-receptor antagonist (1 to 2 mg per kilogram of body weight per day subcutaneously). In 11 patients, anakinra was withdrawn at three months until a flare occurred. The primary end points included changes in scores in a daily diary of symptoms, serum levels of amyloid A and C-reactive protein, and the erythrocyte sedimentation rate from baseline to month 3 and from month 3 until a disease flare.
Results: All 18 patients had a rapid response to anakinra, with disappearance of rash. Diary scores improved (P<0.001) and serum amyloid A (from a median of 174 mg to 8 mg per liter), C-reactive protein (from a median of 5.29 mg to 0.34 mg per deciliter), and the erythrocyte sedimentation rate decreased at month 3 (all P<0.001), and remained low at month 6. Magnetic resonance imaging showed improvement in cochlear and leptomeningeal lesions as compared with baseline. Withdrawal of anakinra uniformly resulted in relapse within days; retreatment led to rapid improvement. There were no drug-related serious adverse events.
Conclusions: Daily injections of anakinra markedly improved clinical and laboratory manifestations in patients with neonatal-onset multisystem inflammatory disease, with or without CIAS1 mutations. (ClinicalTrials.gov number, NCT00069329 [ClinicalTrials.gov].).
Copyright 2006 Massachusetts Medical Society.
Conflict of interest statement
Dr. Stein reports having received consulting and lectures fees from Amgen and Genentech and research support from Amgen and Abbott; Dr. Moore, lecture fees from Amgen; Dr. Vehe, lecture fees from Amgen and research support from Abbott; and Dr. Cole, consulting fees from Abbott and lecture fees from Amgen. Amgen produces and distributes the medication evaluated in this study. No other potential conflict of interest relevant to this article was reported.
Figures
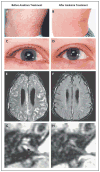
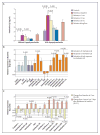
Comment in
-
Anakinra for the treatment of neonatal-onset multisystem inflammatory disease.Nat Clin Pract Rheumatol. 2006 Dec;2(12):646-7. doi: 10.1038/ncprheum0350. Nat Clin Pract Rheumatol. 2006. PMID: 17133246 No abstract available.
References
-
- Prieur AM, Griscelli C. Arthropathy with rash, chronic meningitis, eye lesions, and mental retardation. J Pediatr. 1981;99:79–83. - PubMed
-
- Database of human genes and genetic disorders: OMIM (Online Mendelian Inheritance in Man) Bethesda, Md: National Center for Biotechnology Information; 2006. [Accessed July 14, 2006]. at http://www.ncbi.nlm.nih.gov/entrez/Omim.
-
- Prieur AM, Griscelli C, Lampert F, et al. A chronic, infantile, neurological, cutaneous and articular (CINCA) syndrome: a specific entity analysed in 30 patients. Scand J Rheumatol Suppl. 1987;66:57–68. - PubMed
-
- Aksentijevich I, Nowak M, Mallah M, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum. 2002;46:3340–8. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
