Imaging of cell migration
- PMID: 16900100
- PMCID: PMC1538568
- DOI: 10.1038/sj.emboj.7601227
Imaging of cell migration
Abstract
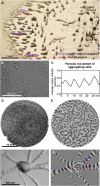
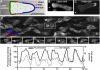
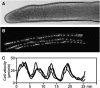
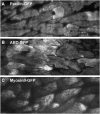
Cell migration is an essential process during many phases of development and adult life. Cells can either migrate as individuals or move in the context of tissues. Movement is controlled by internal and external signals, which activate complex signal transduction cascades resulting in highly dynamic and localised remodelling of the cytoskeleton, cell-cell and cell-substrate interactions. To understand these processes, it will be necessary to identify the critical structural cytoskeletal components, their spatio-temporal dynamics as well as those of the signalling pathways that control them. Imaging plays an increasingly important and powerful role in the analysis of these spatio-temporal dynamics. We will highlight a variety of imaging techniques and their use in the investigation of various aspects of cell motility, and illustrate their role in the characterisation of chemotaxis in Dictyostelium and cell movement during gastrulation in chick embryos in more detail.
Figures



References
-
- Abe T, Early A, Siegert F, Weijer C, Williams J (1994) Patterns of cell movement within the Dictyostelium slug revealed by cell type-specific, surface labeling of living cells. Cell 77: 687–699 - PubMed
-
- Adams SR, Campbell RE, Gross LA, Martin BR, Walkup GK, Yao Y, Llopis J, Tsien RY (2002) New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J Am Chem Soc 124: 6063–6076 - PubMed
-
- Affolter M, Weijer CJ (2005) Signaling to cytoskeletal dynamics during chemotaxis. Dev Cell 9: 19–34 - PubMed
-
- Arai Y, Iwane AH, Wazawa T, Yokota H, Ishii Y, Kataoka T, Yanagida T (2006) Dynamic polymorphism of Ras observed by single molecule FRET is the basis for molecular recognition. Biochem Biophys Res Commun 343: 809–815 - PubMed
-
- Arrieumerlou C, Meyer T (2005) A local coupling model and compass parameter for eukaryotic chemotaxis. Dev Cell 8: 215–227 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

