TTLL7 is a mammalian beta-tubulin polyglutamylase required for growth of MAP2-positive neurites
- PMID: 16901895
- PMCID: PMC3049811
- DOI: 10.1074/jbc.M603984200
TTLL7 is a mammalian beta-tubulin polyglutamylase required for growth of MAP2-positive neurites
Abstract
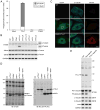
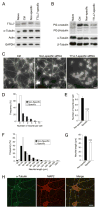
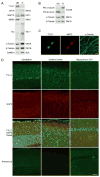
Microtubules form a cytoskeletal framework that influences cell shape and provides structural support for the cell. Microtubules in the nervous system undergo a unique post-translational modification, polyglutamylation of the C termini of their tubulin subunits. The mammalian enzymes that perform beta-tubulin polyglutamylation as well as their physiological functions in the neuronal tissue remain elusive. We report identification of a mammalian polyglutamylase with specificity for beta-tubulin as well as its distribution and function in neurite growth. To identify putative tubulin polyglutamylases, we searched tubulin tyrosine ligase-like (TTLL) proteins for those predominantly expressed in the nervous system. Of 13 TTLL proteins, TTLL7 was transcribed at the highest level in the nervous system. Recombinant TTLL7 catalyzed tubulin polyglutamylation with high preference to beta-tubulin in vitro. When expressed in HEK293T cells, TTLL7 demonstrated specificity for beta-tubulin and not for alpha-tubulin or nucleosome assembly protein 1. Consistent with these findings, knockdown of TTLL7 in a primary culture of superior cervical ganglion neurons caused a loss of polyglutamylated beta-tubulin. Following stimulation of PC12 cells with nerve growth factor to differentiate, the level of TTLL7 increased concomitantly with polyglutamylation of beta-tubulin. Short interference RNA-mediated knockdown of TTLL7 repressed nerve growth factor-stimulated MAP (microtubule-associated protein) 2-positive neurite growth in PC12 cells. Consistent with having a role in the growth of MAP2-positive neurites, TTLL7 accumulated within a MAP2-enriched somatodendritic portion of superior cervical ganglion, as did polyglutamylated beta-tubulin. Anti-TTLL7 antibody revealed that TTLL7 was distributed in a somatodendritic compartment in the mouse brain. These findings indicate that TTLL7 is a beta-tubulin polyglutamylase and is required for the growth of MAP2-positive neurites in PC12 cells.
Figures

Similar articles
-
Recombinant mammalian tubulin polyglutamylase TTLL7 performs both initiation and elongation of polyglutamylation on beta-tubulin through a random sequential pathway.Biochemistry. 2009 Feb 10;48(5):1084-93. doi: 10.1021/bi802047y. Biochemistry. 2009. PMID: 19152315
-
The distinct initiation sites and processing activities of TTLL4 and TTLL7 in glutamylation of brain tubulin.J Biol Chem. 2023 Jul;299(7):104923. doi: 10.1016/j.jbc.2023.104923. Epub 2023 Jun 14. J Biol Chem. 2023. PMID: 37321451 Free PMC article.
-
Distinct roles of α- and β-tubulin polyglutamylation in controlling axonal transport and in neurodegeneration.EMBO J. 2021 Sep 1;40(17):e108498. doi: 10.15252/embj.2021108498. Epub 2021 Jul 26. EMBO J. 2021. PMID: 34309047 Free PMC article.
-
Potential role of tubulin tyrosine ligase-like enzymes in tumorigenesis and cancer cell resistance.Cancer Lett. 2014 Aug 1;350(1-2):1-4. doi: 10.1016/j.canlet.2014.04.022. Epub 2014 May 6. Cancer Lett. 2014. PMID: 24814394 Review.
-
Polyglutamylation: biology and analysis.Amino Acids. 2022 Apr;54(4):529-542. doi: 10.1007/s00726-022-03146-4. Epub 2022 Mar 31. Amino Acids. 2022. PMID: 35357568 Free PMC article. Review.
Cited by
-
Tubulin polyglutamylation is essential for airway ciliary function through the regulation of beating asymmetry.Proc Natl Acad Sci U S A. 2010 Jun 8;107(23):10490-5. doi: 10.1073/pnas.1002128107. Epub 2010 May 24. Proc Natl Acad Sci U S A. 2010. PMID: 20498047 Free PMC article.
-
The Role of Spastin in Axon Biology.Front Cell Dev Biol. 2022 Jul 5;10:934522. doi: 10.3389/fcell.2022.934522. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35865632 Free PMC article. Review.
-
Polyglutamylation: a fine-regulator of protein function? 'Protein Modifications: beyond the usual suspects' review series.EMBO Rep. 2008 Jul;9(7):636-41. doi: 10.1038/embor.2008.114. Epub 2008 Jun 20. EMBO Rep. 2008. PMID: 18566597 Free PMC article. Review.
-
Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions.Nat Rev Mol Cell Biol. 2011 Nov 16;12(12):773-86. doi: 10.1038/nrm3227. Nat Rev Mol Cell Biol. 2011. PMID: 22086369 Review.
-
The tubulin code: molecular components, readout mechanisms, and functions.J Cell Biol. 2014 Aug 18;206(4):461-72. doi: 10.1083/jcb.201406055. J Cell Biol. 2014. PMID: 25135932 Free PMC article. Review.
References
-
- Luduena RF. Int Rev Cytol. 1998;178:207–275. - PubMed
-
- L’Hernault SW, Rosenbaum JL. Biochemistry. 1985;24:473–478. - PubMed
-
- Argarana CE, Barra HS, Caputto R. Mol Cell Biochem. 1978;19:17–21. - PubMed
-
- Cambray-Deakin MA, Burgoyne RD. Cell Motil Cytoskel. 1987;8:284–291. - PubMed
-
- Eddé B, Rossier J, Le Caer JP, Desbruyères E, Gros F, Denoulet P. Science. 1990;247:83–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

