The protein kinase C delta catalytic fragment targets Mcl-1 for degradation to trigger apoptosis
- PMID: 16901898
- PMCID: PMC2205529
- DOI: 10.1074/jbc.M607351200
The protein kinase C delta catalytic fragment targets Mcl-1 for degradation to trigger apoptosis
Abstract
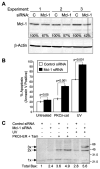
Proteolytic cleavage and subsequent activation of protein kinase C (PKC) delta is required for apoptosis induced by a variety of genotoxic agent, including UV radiation. In addition, overexpression of the constitutively active PKCdelta catalytic fragment (PKCdelta-cat) is sufficient to trigger Bax activation, cytochrome c release, and apoptosis. While PKCdelta is a key apoptotic effector, the downstream target(s) responsible for the mitochondrial apoptotic cascade are not known. We found that expression of the active PKCdelta-cat in HaCaT cells triggers a reduction in the anti-apoptotic protein Mcl-1, similar to UV radiation. The down-regulation of Mcl-1 induced by PKCdelta-cat was not at the mRNA level but was due to decreased protein half-life. Overexpression of Mcl-1 protected HaCaT cells from both UV and PKCdelta-cat-induced apoptosis and blocked the release of cytochrome c from the mitochondria, indicating that Mcl-1 down-regulation was required for apoptosis signaling. Indeed, down-regulation of Mcl-1 with siRNA slightly increased the basal apoptotic rate of HaCaT cells and dramatically sensitized them to UV or PKCdelta-cat-induced apoptosis. HaCaT cells with down-regulated Mcl-1 had higher activated Bax protein, as measured by Bax cross-linking, indicating that Mcl-1 down-regulation is sufficient for Bax activation. Finally, recombinant PKCdelta could phosphorylate Mcl-1 in vitro, identifying Mcl-1 as a direct target for PKCdelta. Overall our results identify Mcl-1 as an important target for PKCdelta-cat that can mediate its pro-apoptotic effects on mitochondria to amplify the apoptotic signaling induced by a wide range of apoptotic stimuli.
Figures



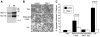

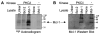
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

