Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia
- PMID: 16901914
- PMCID: PMC2605397
- DOI: 10.1093/brain/awl207
Neuroprotection by PlGF gene-modified human mesenchymal stem cells after cerebral ischaemia
Abstract
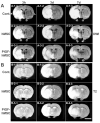
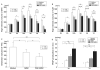
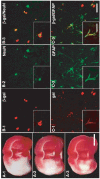
Intravenous delivery of mesenchymal stem cells (MSCs) prepared from adult bone marrow reduces infarction size and ameliorates functional deficits in rat cerebral ischaemia models. Placental growth factor (PlGF) is angiogenic to impaired non-neural tissue. To test the hypothesis that PlGF contributes to the therapeutic benefits of MSC delivery in cerebral ischaemia, we compared the efficacy of systemic delivery of human MSCs (hMSCs) and hMSCs transfected with a fibre-mutant F/RGD adenovirus vector with a PlGF gene (PlGF-hMSCs). A permanent middle cerebral artery occlusion (MCAO) was induced by intraluminal vascular occlusion with a microfilament. hMSCs and PlGF-hMSCs were intravenously injected into the rats 3 h after MCAO. Lesion size was assessed at 3 and 6 h, and 1, 3, 4 and 7 days using MR imaging and histology. Functional outcome was assessed using the limb placement test and the treadmill stress test. Both hMSCs and PlGF-hMSCs reduced lesion volume, induced angiogenesis and elicited functional improvement compared with the control sham group, but the effect was greater in the PlGF-hMSC group. Enzyme-linked immunosorbent assay of the infarcted hemisphere revealed an increase in PlGF in both hMSC groups, but a greater increase in the PlGF-hMSC group. These data support the hypothesis that PlGF contributes to neuroprotection and angiogenesis in cerebral ischaemia, and cellular delivery of PlGF to the brain can be achieved by intravenous delivery of hMSCs.
Figures





Similar articles
-
I.V. infusion of brain-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in adult rat.Neuroscience. 2005;136(1):161-9. doi: 10.1016/j.neuroscience.2005.06.062. Epub 2005 Oct 17. Neuroscience. 2005. PMID: 16229956 Free PMC article.
-
Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia.J Cereb Blood Flow Metab. 2008 Feb;28(2):329-40. doi: 10.1038/sj.jcbfm.9600527. Epub 2007 Jul 18. J Cereb Blood Flow Metab. 2008. PMID: 17637706 Free PMC article.
-
Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat.J Neurosci Res. 2006 Nov 15;84(7):1495-504. doi: 10.1002/jnr.21056. J Neurosci Res. 2006. PMID: 16998918 Free PMC article.
-
Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia.Exp Neurol. 2009 Mar;216(1):47-55. doi: 10.1016/j.expneurol.2008.11.010. Epub 2008 Nov 27. Exp Neurol. 2009. PMID: 19094989
-
Neuroprotection by mesenchymal stem cell (MSC) administration is enhanced by local cooling infusion (LCI) in ischemia.Brain Res. 2019 Dec 1;1724:146406. doi: 10.1016/j.brainres.2019.146406. Epub 2019 Aug 24. Brain Res. 2019. PMID: 31454517 Review.
Cited by
-
Brain Aging and Regeneration after Injuries: an Organismal approach.Aging Dis. 2011 Feb;2(1):64-79. Epub 2011 Sep 20. Aging Dis. 2011. PMID: 22396867 Free PMC article.
-
MSC based gene delivery methods and strategies improve the therapeutic efficacy of neurological diseases.Bioact Mater. 2022 Nov 30;23:409-437. doi: 10.1016/j.bioactmat.2022.11.007. eCollection 2023 May. Bioact Mater. 2022. PMID: 36474656 Free PMC article. Review.
-
Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice.Brain. 2012 Nov;135(Pt 11):3298-310. doi: 10.1093/brain/aws259. Brain. 2012. PMID: 23169920 Free PMC article.
-
Stem cells in human neurodegenerative disorders--time for clinical translation?J Clin Invest. 2010 Jan;120(1):29-40. doi: 10.1172/JCI40543. J Clin Invest. 2010. PMID: 20051634 Free PMC article. Review.
-
Achieving stable human stem cell engraftment and survival in the CNS: is the future of regenerative medicine immunodeficient?Regen Med. 2011 May;6(3):367-406. doi: 10.2217/rme.11.22. Regen Med. 2011. PMID: 21548741 Free PMC article.
References
-
- Autiero M, Luttun A, Tjwa M, Carmeliet P. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost. 2003;1:1356–70. - PubMed
-
- Beck H, Acker T, Puschel AW, Fujisawa H, Carmeliet P, Plate KH. Cell type-specific expression of neuropilins in an MCA-occlusion model in mice suggests a potential role in post-ischemic brain remodeling. J Neuropathol Exp Neurol. 2002;61:339–50. - PubMed
-
- Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5—triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–8. - PubMed
-
- Carmeliet P, Moons L, Luttun A, Vincenti V, Compernolle V, De Mol M, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med. 2001;7:575–83. - PubMed
-
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001a;189:49–57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

