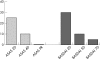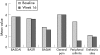No efficacy of subcutaneous methotrexate in active ankylosing spondylitis: a 16-week open-label trial
- PMID: 16901959
- PMCID: PMC1856012
- DOI: 10.1136/ard.2006.054098
No efficacy of subcutaneous methotrexate in active ankylosing spondylitis: a 16-week open-label trial
Abstract
Objective: To examine the potential therapeutic effect of methotrexate 20 mg given weekly as subcutaneous injections to 20 patients with ankylosing spondylitis refractory to non-steroidal antirheumatic drugs.
Patients and methods: 20 patients with ankylosing spondylitis, a mean Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score of 5.6 (range 4-9.3) and predominantly axial manifestations were treated with weekly 15 mg methotrexate subcutaneously for 4 weeks, which was then increased to 20 mg subcutaneously for the next 12 weeks. Clinical outcome assessments included, among others, BASDAI score physical function, spinal mobility, patients' and physicians' global assessment (visual analogue scale), peripheral joint assessment, quality of life (Short Form 36) and C reactive protein. The primary end point of the study was a 20% improvement on the ASsessments in Ankylosing Spondylitis (ASAS 20) scale.
Results: Using an intention-to-treat analysis, ASAS 20 was achieved in only 25% of patients. An ASAS 40 response was achieved in 10% of patients, and no patient reached an ASAS 70 response or the ASAS criteria for partial remission. For the mean BASDAI score, no change was observed between baseline and week 16 (baseline 5.6 v week 16, 5.6). No improvement was observed in any of the clinical parameters or C reactive protein, except a small but non-significant decrease in the number of swollen joints.
Conclusions: In this open study, methotrexate did not show any benefit for axial manifestations in patients with active ankylosing spondylitis beyond the expected placebo response.
Conflict of interest statement
Competing interests: None declared.
Comment in
-
Is methotrexate effective for the treatment of ankylosing spondylitis?Nat Clin Pract Rheumatol. 2007 Sep;3(9):490-1. doi: 10.1038/ncprheum0566. Epub 2007 Jul 31. Nat Clin Pract Rheumatol. 2007. PMID: 17667906 No abstract available.
References
-
- Breban M, Ravaud P, Claudepierre P, Baron G, Hudry C, Euller‐Ziegler L.et al No superiority of infliximab (INF) + methotrexate (MTX) over INF alone in the treatment of ankylosing spondylitis (AS): results of a one‐year randomized prospective study. Arthritis Rheum 200552(Suppl)S214
-
- Heiberg M S, Nordvag B Y, Mikkelsen K, Rodevand E, Kaufmann C, Mowinckel P.et al The comparative effectiveness of tumor necrosis factor‐blocking agents in patients with rheumatoid arthritis and patients with ankylosing spondylitis: a six‐month, longitudinal, observational, multicenter study. Arthritis Rheum 2005522506–2511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials