Silica-directed mast cell activation is enhanced by scavenger receptors
- PMID: 16902192
- PMCID: PMC1899302
- DOI: 10.1165/rcmb.2006-0197OC
Silica-directed mast cell activation is enhanced by scavenger receptors
Abstract
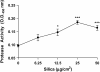
Inhalation of crystalline silica results in pulmonary fibrosis and silicosis. It has been suggested that mast cells play a role in these conditions. How mast cells would influence pathology is unknown. We thus explored mast cell interactions with silica in vitro and in B6.Cg-kit(W-sh) mast cell-deficient mice. B6.Cg-kit(W-sh) mice did not develop inflammation or significant collagen deposition after instillation of silica, while C57Bl/6 wild-type mice did have these findings. Given this supporting evidence of a role for mast cells in the development of silicosis, we examined the ability of silica to activate mouse bone marrow-derived mast cells (BMMC), including degranulation (beta-hexosaminidase release); production of reactive oxygen species (ROS) and inflammatory mediators; and the effects of silica on Fc epsilon RI-dependent activation. Silica did not induce mast cell degranulation. However, TNF-alpha, IL-13, monocyte chemotactic protein-1, protease activity, and production of ROS were dose-dependently increased after silica exposure, and production was enhanced after Fc epsilon RI stimulation. This mast cell activation was inhibited by anti-inflammatory compounds. As silica mediates some effects in macrophages through scavenger receptors (SRs), we first determined that mast cells express scavenger receptors; then explored the involvement of SR-A and macrophage receptor with colleagenous structure (MARCO). Silica-induced ROS formation, apoptosis, and TNF-alpha production were reduced in BMMC obtained from SR-A, MARCO, and SR-A/MARCO knockout mice. These findings demonstrate that silica directs mast cell production of inflammatory mediators, in part through SRs, providing insight into critical events in the pathogenesis and potential therapeutic targets in silicosis.
Figures




References
-
- American thoracic society committee of the scientific assembly on environmental and occupational health. Adverse effects of crystalline silica exposure. Am J Respir Crit Care Med 1997;155:761–768. - PubMed
-
- Bang KM, Mazurek JM, Attfield MD. Silicosis mortality, prevention, and control–United States, 1968–2002. MMWR Morb Mortal Wkly Rep 2005;54:401–405. - PubMed
-
- Kopinski P, Czunko P, Soja J, Lackowska B, Gil K, Jedynak U, Szczeklik J, Sladek K, Chlap Z. Pneumonol Alergol Pol 2000;68:109–119. (cytoimmunologic changes in material obtained from bronchoalveolar lavage (bal) in asymptomatic individuals chronically exposed to silica dust). - PubMed
-
- Hamada H, Vallyathan V, Cool CD, Barker E, Inoue Y, Newman LS. Mast cell basic fibroblast growth factor in silicosis. Am J Respir Crit Care Med 2000;161:2026–2034. - PubMed
-
- Suzuki H, Kurihara Y, Takeya M, Kamada N, Kataoka M, Jishage K, Ueda O, Sakaguchi H, Higashi T, Suzuki T, et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 1997;386:292–296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

