Macrophages inhibit neovascularization in a murine model of age-related macular degeneration
- PMID: 16903779
- PMCID: PMC1539093
- DOI: 10.1371/journal.pmed.0030310
Macrophages inhibit neovascularization in a murine model of age-related macular degeneration
Abstract
Background: Age-related macular degeneration (AMD) is the leading cause of blindness in people over 50 y of age in at least three continents. Choroidal neovascularization (CNV) is the process by which abnormal blood vessels develop underneath the retina. CNV develops in 10% of patients with AMD but accounts for up to 90% of the blindness from AMD. Although the precise etiology of CNV in AMD remains unknown, the macrophage component of the inflammatory response, which has been shown to promote tumor growth and support atherosclerotic plaque formation, is thought to stimulate aberrant angiogenesis in blinding eye diseases. The current theory is that macrophage infiltration promotes the development of neovascularization in CNV.
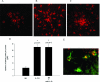
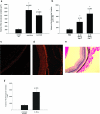
Methods and findings: We examined the role of macrophages in a mouse model of CNV. IL-10(-/-) mice, which have increased inflammation in response to diverse stimuli, have significantly reduced CNV with increased macrophage infiltrates compared to wild type. Prevention of macrophage entry into the eye promoted neovascularization while direct injection of macrophages significantly inhibited CNV. Inhibition by macrophages was mediated by the TNF family death molecule Fas ligand (CD95-ligand).
Conclusions: Immune vascular interactions can be highly complex. Normal macrophage function is critical in controlling pathologic neovascularization in the eye. IL-10 regulates macrophage activity in the eye and is an attractive therapeutic target in order to suppress or inhibit CNV in AMD that can otherwise lead to blindness.
Conflict of interest statement
Figures



Comment in
-
Is IL-10 a good target to inhibit choroidal neovascularisation in age-related macular disease?PLoS Med. 2006 Aug;3(8):e364. doi: 10.1371/journal.pmed.0030364. PLoS Med. 2006. PMID: 16903781 Free PMC article.
References
-
- Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD, et al. Macrophage receptors and immune recognition. Annu Rev Immunol. 2005;23:901–944. - PubMed
-
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. - PubMed
-
- Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J. Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3578–3585. - PubMed
-
- Espinosa-Heidmann DG, Suner IJ, Hernandez EP, Monroy D, Csaky KG, et al. Macrophage depletion diminishes lesion size and severity in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3586–3592. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

