Carbocyclic pyrimidine nucleosides as inhibitors of S-adenosylhomocysteine hydrolase
- PMID: 16904326
- PMCID: PMC1702506
- DOI: 10.1016/j.bmc.2006.07.052
Carbocyclic pyrimidine nucleosides as inhibitors of S-adenosylhomocysteine hydrolase
Abstract
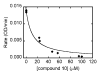
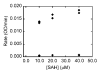
The design, synthesis, and unexpected inhibitory activity against S-adenosyl-homocysteine (SAH) hydrolase (SAHase, EC 3.3.1.1) for a series of truncated carbocyclic pyrimidine nucleoside analogues is presented. Of the four nucleosides obtained, 10 was found to be active with a Ki value of 5.0 microM against SAHase.
Figures
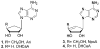




Similar articles
-
Synthetic strategies toward carbocyclic purine-pyrimidine hybrid nucleosides.Bioorg Med Chem. 2009 Aug 1;17(15):5520-5. doi: 10.1016/j.bmc.2009.06.039. Epub 2009 Jun 23. Bioorg Med Chem. 2009. PMID: 19592260 Free PMC article.
-
Potential inhibitors of S-adenosylmethionine-dependent methyltransferases. 9. 2',3'-Dialdehyde derivatives of carbocyclic purine nucleosides as inhibitors of S-adenosylhomocysteine hydrolase.J Med Chem. 1985 Apr;28(4):471-7. doi: 10.1021/jm00382a015. J Med Chem. 1985. PMID: 3981539
-
Adenosine analogues as substrates and inhibitors of S-adenosylhomocysteine hydrolase.Biochemistry. 1981 Jan 6;20(1):110-5. doi: 10.1021/bi00504a019. Biochemistry. 1981. PMID: 7470463
-
[S-adenosyl-L-homocysteine hydrolase as an attractive target for antimicrobial drugs].Yakugaku Zasshi. 2007 Jun;127(6):977-82. doi: 10.1248/yakushi.127.977. Yakugaku Zasshi. 2007. PMID: 17541248 Review. Japanese.
-
Carbocyclic nucleosides.Med Res Rev. 1986 Jan-Mar;6(1):1-40. doi: 10.1002/med.2610060102. Med Res Rev. 1986. PMID: 3512934 Review. No abstract available.
Cited by
-
"Reverse" carbocyclic fleximers: synthesis of a new class of adenosine deaminase inhibitors.Nucleosides Nucleotides Nucleic Acids. 2013;32(3):137-54. doi: 10.1080/15257770.2013.771187. Nucleosides Nucleotides Nucleic Acids. 2013. PMID: 23473101 Free PMC article.
-
Synthesis of 5'-functionalized nucleosides: S-Adenosylhomocysteine analogues with the carbon-5' and sulfur atoms replaced by a vinyl or halovinyl unit.Bioorg Med Chem. 2008 May 15;16(10):5424-33. doi: 10.1016/j.bmc.2008.04.017. Epub 2008 Apr 12. Bioorg Med Chem. 2008. PMID: 18457953 Free PMC article.
-
Carbocyclic thymidine analogues for use as potential therapeutic agents.Nucleosides Nucleotides Nucleic Acids. 2009 May;28(5):633-41. doi: 10.1080/15257770903091920. Nucleosides Nucleotides Nucleic Acids. 2009. PMID: 20183606 Free PMC article.
-
Synthetic strategies toward carbocyclic purine-pyrimidine hybrid nucleosides.Bioorg Med Chem. 2009 Aug 1;17(15):5520-5. doi: 10.1016/j.bmc.2009.06.039. Epub 2009 Jun 23. Bioorg Med Chem. 2009. PMID: 19592260 Free PMC article.
-
Significance and biological importance of pyrimidine in the microbial world.Int J Med Chem. 2014;2014:202784. doi: 10.1155/2014/202784. Epub 2014 Mar 23. Int J Med Chem. 2014. PMID: 25383216 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

