Increased efficacy of HIV-1 neutralization by antibodies at low CCR5 surface concentration
- PMID: 16904645
- PMCID: PMC2268024
- DOI: 10.1016/j.bbrc.2006.07.163
Increased efficacy of HIV-1 neutralization by antibodies at low CCR5 surface concentration
Abstract
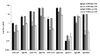
It has been observed that some antibodies, including the CD4-induced (CD4i) antibody IgG X5 and the gp41-specific antibody IgG 2F5, exhibit higher neutralizing activity in PBMC-based assays than in cell line based assays [J.M. Binley, T. Wrin, B. Korber, M.B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C.J. Petropoulos, D.R. Burton, Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies, J. Virol. 78 (2004) 13232-13252]. It has been hypothesized that the lower CCR5 concentration on the surface of the CD4 T lymphocytes compared to that on cell lines used for the neutralization assays could be a contributing factor to the observed differences in neutralizing activity. To test this hypothesis and to further elucidate the contribution of CCR5 concentration differences on antibody neutralizing activity, we used a panel of HeLa cell lines with well-defined and differential surface concentrations of CCR5 and CD4 in a pseudovirus-based assay. We observed that the CCR5 cell surface concentration but not the CD4 concentration had a significant effect on the inhibitory activity of X5 and several other CD4i antibodies including 17b and m9, as well as that of the gp41-specifc antibodies 2F5 and 4E10 but not on that of the CD4 binding site antibody (CD4bs), b12. The 50% inhibitory concentration (IC50) decreased up to two orders of magnitude in cell lines with low CCR5 concentration corresponding to that in CD4 T cells used in PBMC-based assays (about 10(3) per cell) compared to cell lines with high CCR5 concentration (about 10(4) or more). Our results suggest that the CCR5 cell surface concentration could be a contributing factor to the high neutralizing activities of some antibodies in PBMC-based-assays but other factors could also play an important role. These findings could have implications for development of vaccine immunogens based on the epitopes of X5 and other CD4i antibodies, for elucidation of the mechanisms of HIV-1 neutralization by antibodies, and for design of novel therapeutic approaches.
Figures







References
-
- Smith AE, Helenius A. How viruses enter animal cells. Science. 2004;304:237–242. - PubMed
-
- Reeves JD, Gallo SA, Ahmad N, Miamidian JL, Harvey PE, Sharron M, Pohlmann S, Sfakianos JN, Derdeyn CA, Blumenthal R, Hunter E, Doms RW. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc.Natl.Acad.Sci.U.S.A. 2002;99:16249–16254. - PMC - PubMed
-
- Moulard M, Phogat SK, Shu Y, Labrijn AF, Xiao XD, Binley JM, Zhang MY, Sidorov IA, Broder CC, Robinson J, Parren PWHI, Burton DR, Dimitrov DS. Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120-CD4-CCR5 complexes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6913–6918. - PMC - PubMed
-
- Labrijn AF, Poignard P, Raja A, Zwick MB, Delgado K, Franti M, Binley J, Vivona V, Grundner C, Huang CC, Venturi M, Petropoulos CJ, Wrin T, Dimitrov DS, Robinson J, Kwong PD, Wyatt RT, Sodroski J, Burton DR. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J.Virol. 2003;77:10557–10565. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

