Biochemical and functional characterization of three activated macrophage populations
- PMID: 16905575
- PMCID: PMC2642590
- DOI: 10.1189/jlb.0406249
Biochemical and functional characterization of three activated macrophage populations
Abstract
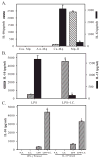
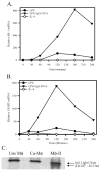
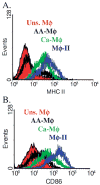
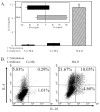
We generated three populations of macrophages (Mphi) in vitro and characterized each. Classically activated Mphi (Ca-Mphi) were primed with IFN-gamma and stimulated with LPS. Type II-activated Mphi (Mphi-II) were similarly primed but stimulated with LPS plus immune complexes. Alternatively activated Mphi (AA-Mphi) were primed overnight with IL-4. Here, we present a side-by-side comparison of the three cell types. We focus primarily on differences between Mphi-II and AA-Mphi, as both have been classified as M2 Mphi, distinct from Ca-Mphi. We show that Mphi-II more closely resemble Ca-Mphi than they are to AA-Mphi. Mphi-II and Ca-Mphi, but not AA-Mphi, produce high levels of NO and have low arginase activity. AA-Mphi express FIZZ1, whereas neither Mphi-II nor Ca-Mphi do. Mphi-II and Ca-Mphi express relatively high levels of CD86, whereas AA-Mphi are virtually devoid of this costimulatory molecule. Ca-Mphi and Mphi-II are efficient APC, whereas AA-Mphi fail to stimulate efficient T cell proliferation. The differences between Ca-Mphi and Mphi-II are more subtle. Ca-Mphi produce IL-12 and give rise to Th1 cells, whereas Mphi-II produce high levels of IL-10 and thus, give rise to Th2 cells secreting IL-4 and IL-10. Mphi-II express two markers that may be used to identify them in tissue. These are sphingosine kinase-1 and LIGHT (TNF superfamily 14). Thus, Ca-Mphi, Mphi-II, and AA-Mphi represent three populations of cells with different biological functions.
Figures




References
-
- Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. 2002;169:2253–2263. - PubMed
-
- David JR. Macrophage activation by lymphocyte mediators. Fed Proc. 1975;34:1730–1736. - PubMed
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

