Heterogeneity of early intermediates in cell-liposome fusion mediated by influenza hemagglutinin
- PMID: 16905609
- PMCID: PMC1614502
- DOI: 10.1529/biophysj.106.088005
Heterogeneity of early intermediates in cell-liposome fusion mediated by influenza hemagglutinin
Abstract
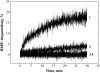
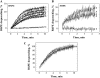
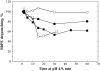
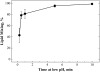
To explore early intermediates in membrane fusion mediated by influenza virus hemagglutinin (HA) and their dependence on the composition of the target membrane, we studied lipid mixing between HA-expressing cells and liposomes containing phosphatidylcholine (PC) with different hydrocarbon chains. For all tested compositions, our results indicate the existence of at least two types of intermediates, which differ in their lifetimes. The composition of the target membrane affects the stability of fusion intermediates at a stage before lipid mixing. For less fusogenic distearoyl PC-containing liposomes at 4 degrees C, some of the intermediates inactivate, and no intermediates advance to lipid mixing. Fusion intermediates that formed for the more fusogenic dioleoyl PC-containing liposomes did not inactivate and even yielded partial lipid mixing at 4 degrees C. Thus, a more fusogenic target membrane effectively blocks nonproductive release of the conformational energy of HA. Even for the same liposome composition, HA forms two types of fusion intermediates, dissimilar in their stability and propensity to fuse. This diversity of fusion intermediates emphasizes the importance of local membrane composition and local protein concentration in fusion of heterogeneous biological membranes.
Figures


Similar articles
-
Liposome composition effects on lipid mixing between cells expressing influenza virus hemagglutinin and bound liposomes.Arch Biochem Biophys. 2005 Jul 15;439(2):211-21. doi: 10.1016/j.abb.2005.05.010. Arch Biochem Biophys. 2005. PMID: 15963452
-
The 1-127 HA2 construct of influenza virus hemagglutinin induces cell-cell hemifusion.Biochemistry. 2001 Jul 27;40(28):8378-86. doi: 10.1021/bi010466+. Biochemistry. 2001. PMID: 11444985
-
The pathway of membrane fusion catalyzed by influenza hemagglutinin: restriction of lipids, hemifusion, and lipidic fusion pore formation.J Cell Biol. 1998 Mar 23;140(6):1369-82. doi: 10.1083/jcb.140.6.1369. J Cell Biol. 1998. PMID: 9508770 Free PMC article.
-
Deployment of membrane fusion protein domains during fusion.Cell Biol Int. 2000;24(11):819-38. doi: 10.1006/cbir.2000.0632. Cell Biol Int. 2000. PMID: 11067767 Review.
-
Membrane fusion mechanisms: the influenza hemagglutinin paradigm and its implications for intracellular fusion.Traffic. 2000 Aug;1(8):598-604. doi: 10.1034/j.1600-0854.2000.010803.x. Traffic. 2000. PMID: 11208147 Review.
Cited by
-
Influence of calcium on lipid mixing mediated by influenza hemagglutinin.Arch Biochem Biophys. 2007 Sep 1;465(1):101-8. doi: 10.1016/j.abb.2007.05.005. Epub 2007 May 25. Arch Biochem Biophys. 2007. PMID: 17585869 Free PMC article.
-
An allosteric rheostat in HIV-1 gp120 reduces CCR5 stoichiometry required for membrane fusion and overcomes diverse entry limitations.J Mol Biol. 2007 Nov 16;374(1):64-79. doi: 10.1016/j.jmb.2007.09.016. Epub 2007 Sep 12. J Mol Biol. 2007. PMID: 17920626 Free PMC article.
References
-
- White, J. M. 1995. Membrane fusion: The influenza paradigm. Cold Spring Harb. Symp. Quant. Biol. 60:581–588. - PubMed
-
- Skehel, J. J., and D. C. Wiley. 2000. Receptor binding and membrane fusion in virus entry: The influenza hemagglutinin. Annu. Rev. Biochem. 69:531–569. - PubMed
-
- Tamm, L. K., J. Crane, and V. Kiessling. 2003. Membrane fusion: a structural perspective on the interplay of lipids and proteins. Curr. Opin. Struct. Biol. 13:453–466. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

