Cytochrome c-d regulates developmental apoptosis in the Drosophila retina
- PMID: 16906130
- PMCID: PMC1559679
- DOI: 10.1038/sj.embor.7400773
Cytochrome c-d regulates developmental apoptosis in the Drosophila retina
Abstract
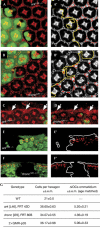
The role of cytochrome c (Cyt c) in caspase activation has largely been established from mammalian cell-culture studies, but much remains to be learned about its physiological relevance in situ. The role of Cyt c in invertebrates has been subject to considerable controversy. The Drosophila genome contains distinct cyt c genes: cyt c-p and cyt c-d. Loss of cyt c-p function causes embryonic lethality owing to a requirement of the gene for mitochondrial respiration. By contrast, cyt c-d mutants are viable but male sterile. Here, we show that cyt c-d regulates developmental apoptosis in the pupal eye. cyt c-d mutant retinas show a profound delay in the apoptosis of superfluous interommatidial cells and perimeter ommatidial cells. Furthermore, there is no apoptosis in mutant retinal tissues for the Drosophila homologues of apoptotic protease-activating factor 1 (Ark) and caspase 9 (Dronc). In addition, we found that cyt c-d--as with ark and dronc-regulates scutellar bristle number, which is known to depend on caspase activity. Collectively, our results indicate a role of Cyt c in caspase regulation of Drosophila somatic cells.
Figures



References
-
- Akdemir F et al. (2006) Autophagy occurs upstream or parallel to the apoptosome during histolytic cell death. Development 133: 1457–1465 - PubMed
-
- Arama E, Agapite J, Steller H (2003) Caspase activity and a specific cytochrome c are required for sperm differentiation in Drosophila. Dev Cell 4: 687–697 - PubMed
-
- Cagan RL, Ready DF (1989) The emergence of order in the Drosophila pupal retina. Dev Biol 136: 346–362 - PubMed
-
- Chew SK, Akdemir F, Chen P, Lu WJ, Mills K, Daish T, Kumar S, Rodriguez A, Abrams JM (2004) The apical caspase dronc governs programmed and unprogrammed cell death in Drosophila. Dev Cell 7: 897–907 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

