Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry
- PMID: 16906426
- PMCID: PMC3906709
- DOI: 10.1007/s00401-006-0127-z
Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry
Abstract
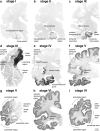
Assessment of Alzheimer's disease (AD)-related neurofibrillary pathology requires a procedure that permits a sufficient differentiation between initial, intermediate, and late stages. The gradual deposition of a hyperphosphorylated tau protein within select neuronal types in specific nuclei or areas is central to the disease process. The staging of AD-related neurofibrillary pathology originally described in 1991 was performed on unconventionally thick sections (100 mum) using a modern silver technique and reflected the progress of the disease process based chiefly on the topographic expansion of the lesions. To better meet the demands of routine laboratories this procedure is revised here by adapting tissue selection and processing to the needs of paraffin-embedded sections (5-15 mum) and by introducing a robust immunoreaction (AT8) for hyperphosphorylated tau protein that can be processed on an automated basis. It is anticipated that this revised methodological protocol will enable a more uniform application of the staging procedure.
Figures




References
-
- Alafuzoff I, Pikkarainen M, Al-Sarraj S, Arzberger T, Bell J, Bodi I, Bogdanovic N, Budka H, Bugiani O, Ferrer I, Gelpi E, Gaiccone G, Graeber MB, Hauw JJ, Kamphorst W, King A, Kopp N, Korkolopolou P, Kovacs GG, Meyronet D, Marchi P, Patsouris E, Preusser M, Ravid R, Roggendorf W, Seilhean D, Streichneberger N, Thal DR, BNE consortium, Kretzschmar H (2006) Inter-laboratory comparison of assessments of AD-related lesions. A study of the BrainNet Europe consortium. J Neuropathol Exp Neurol 65 (in press) - PubMed
-
- Alzheimer A. Über einen eigenartigen schweren Erkrankungsprozeß der Hirnrinde. Neurolog Centralbl. 1906;23:1129–1136.
-
- Alzheimer A. Über eigenartige Krankheitsfälle des späteren Alters. Z ges Neurol Psychiatr. 1911;4:356–385.
-
- Arnold SE, Hyman BT, Flory J, Damasio AR, van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1:103–116. - PubMed
-
- Arriagada PV, Growdon J, Hedley-Whyte E, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992a;42:631–639. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

