The potential risks of nanomaterials: a review carried out for ECETOC
- PMID: 16907977
- PMCID: PMC1584248
- DOI: 10.1186/1743-8977-3-11
The potential risks of nanomaterials: a review carried out for ECETOC
Abstract
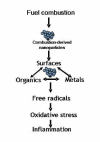
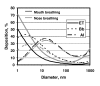
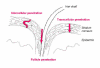
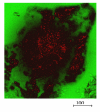
During the last few years, research on toxicologically relevant properties of engineered nanoparticles has increased tremendously. A number of international research projects and additional activities are ongoing in the EU and the US, nourishing the expectation that more relevant technical and toxicological data will be published. Their widespread use allows for potential exposure to engineered nanoparticles during the whole lifecycle of a variety of products. When looking at possible exposure routes for manufactured Nanoparticles, inhalation, dermal and oral exposure are the most obvious, depending on the type of product in which Nanoparticles are used. This review shows that (1) Nanoparticles can deposit in the respiratory tract after inhalation. For a number of nanoparticles, oxidative stress-related inflammatory reactions have been observed. Tumour-related effects have only been observed in rats, and might be related to overload conditions. There are also a few reports that indicate uptake of nanoparticles in the brain via the olfactory epithelium. Nanoparticle translocation into the systemic circulation may occur after inhalation but conflicting evidence is present on the extent of translocation. These findings urge the need for additional studies to further elucidate these findings and to characterize the physiological impact. (2) There is currently little evidence from skin penetration studies that dermal applications of metal oxide nanoparticles used in sunscreens lead to systemic exposure. However, the question has been raised whether the usual testing with healthy, intact skin will be sufficient. (3) Uptake of nanoparticles in the gastrointestinal tract after oral uptake is a known phenomenon, of which use is intentionally made in the design of food and pharmacological components. Finally, this review indicates that only few specific nanoparticles have been investigated in a limited number of test systems and extrapolation of this data to other materials is not possible. Air pollution studies have generated indirect evidence for the role of combustion derived nanoparticles (CDNP) in driving adverse health effects in susceptible groups. Experimental studies with some bulk nanoparticles (carbon black, titanium dioxide, iron oxides) that have been used for decades suggest various adverse effects. However, engineered nanomaterials with new chemical and physical properties are being produced constantly and the toxicity of these is unknown. Therefore, despite the existing database on nanoparticles, no blanket statements about human toxicity can be given at this time. In addition, limited ecotoxicological data for nanomaterials precludes a systematic assessment of the impact of Nanoparticles on ecosystems.
Figures




References
-
- Schmidt G, Decker M, Ernst H, Fuchs H, Grunwald W, Grunwald A, et al. A definiton of nanotechnology. Bad Neuenahr; 2003. Small dimensions and material properties. Europaische Akademie Graue Reihe; p. 134. Ref Type: Generic.
-
- The Royal Society and the Royal Academy of Engineering Nanoscience and nanotechnologies: opportunities and uncertainties. R. 2004.
LinkOut - more resources
Full Text Sources
Other Literature Sources

