Induction of transcription factor Egr-1 gene expression in astrocytoma cells by Murine coronavirus infection
- PMID: 16908043
- PMCID: PMC1851928
- DOI: 10.1016/j.virol.2006.07.012
Induction of transcription factor Egr-1 gene expression in astrocytoma cells by Murine coronavirus infection
Abstract
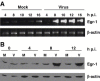
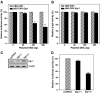
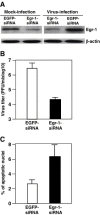
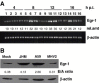
Mouse hepatitis virus (MHV) causes encephalitis and demyelination in the central nervous system (CNS) of susceptible rodents. Astrocytes are one of the major targets for MHV infection in the CNS, and respond to MHV infection by expressing diverse molecules that may contribute to CNS pathogenesis. Here we characterized the activation of an immediate-early transcription factor Egr-1 by MHV infection in an astrocytoma cell line. We found that the expression of Egr-1 was dramatically increased following virus infection. Using various inhibitors of mitogen-activated protein kinases, we identified that the extracellular signal-regulated kinases 1/2 were involved in the activation of Egr-1 transcription by MHV infection. Experiments with ultraviolet light-inactivated virus revealed that the induction of Egr-1 did not require virus replication and was likely mediated during cell entry. We further found that over-expression of Egr-1 suppressed the expression of BNip3, a pro-apoptotic member of the Bcl-2 family. This finding may provide an explanation for our previously observed down-regulation of BNip3 by MHV infection in astrocytoma cells (Cai, Liu, Yu, and Zhang, Virology 316:104-115, 2003). Furthermore, knockdown of Egr-1 by an siRNA inhibited MHV propagation, suggesting the biological relevance of Egr-1 induction to virus replication. In addition, the persistence/demylinating-positive strains (JHM and A59) induced Egr-1 expression, whereas the persistence/demylinating-negative strain (MHV-2) did not. These results indicate a correlation between the ability of MHVs to induce Egr-1 expression and their ability to cause demyelination in the CNS, which may suggest a potential role for the induction of Egr-1 in viral pathogenesis.
Figures



References
-
- Baron V., De Gregorio G., Krones-Herzig A., Virolle T., Calogero A., Urcis R., Mercola D. Inhibition of Egr-1 expression reverses transformation of prostate cancer cells in vitro and in vivo. Oncogene. 2003;22:4194–4204. - PubMed
-
- Baron V., Duss S., Rhim J., Mercola D. Antisense to the early growth response-1 gene (Egr-1) inhibits prostate tumor development in TRAMP mice. Ann. N. Y. Acad. Sci. 2003;1002:197–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

