Perturbing integrin function inhibits microtubule growth from centrosomes, spindle assembly, and cytokinesis
- PMID: 16908668
- PMCID: PMC2064255
- DOI: 10.1083/jcb.200603069
Perturbing integrin function inhibits microtubule growth from centrosomes, spindle assembly, and cytokinesis
Abstract
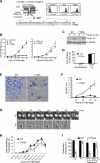
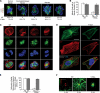
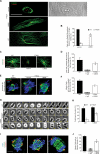
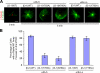
In many mammalian cell types, integrin-mediated cell-matrix adhesion is required for the G1-S transition of the cell cycle. As cells approach mitosis, a dramatic remodeling of their cytoskeleton accompanies dynamic changes in matrix adhesion, suggesting a mechanistic link. However, the role of integrins in cell division remains mostly unexplored. Using two cellular systems, we demonstrate that a point mutation in the beta1 cytoplasmic domain (beta1 tail) known to decrease integrin activity supports entry into mitosis but inhibits the assembly of a radial microtubule array focused at the centrosome during interphase, the formation of a bipolar spindle at mitosis and cytokinesis. These events are restored by externally activating the mutant integrin with specific antibodies. This is the first demonstration that the integrin beta1 tail can regulate centrosome function, the assembly of the mitotic spindle, and cytokinesis.
Figures

References
-
- Assoian, R.K., and M.A. Schwartz. 2001. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev. 11:48–53. - PubMed
-
- Ben-Ze'ev, A., and A. Raz. 1981. Multinucleation and inhibition of cytokinesis in suspended cells: reversal upon reattachment to a substrate. Cell. 26:107–115. - PubMed
-
- Bodeau, A.L., A.L. Berrier, A.M. Mastrangelo, R. Martinez, and S.E. LaFlamme. 2001. A functional comparison of mutations in integrin beta cytoplasmic domains: effects on the regulation of tryosine phosphorylation, cell spreading, cell attachment and beta1 integrin conformation. J. Cell Sci. 114:2795–2807. - PubMed
-
- Doxsey, S. 2001. Re-evaluating centrosome function. Nat. Rev. Mol. Cell Biol. 2:688–698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

