Efficient generation of retinal progenitor cells from human embryonic stem cells
- PMID: 16908856
- PMCID: PMC1568922
- DOI: 10.1073/pnas.0601990103
Efficient generation of retinal progenitor cells from human embryonic stem cells
Abstract
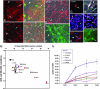
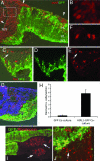
The retina is subject to degenerative conditions, leading to blindness. Although retinal regeneration is robust in lower vertebrates, regeneration does not occur in the adult mammalian retina. Thus, we have developed efficient methods for deriving retinal neurons from human embryonic stem (hES) cells. Under appropriate culture conditions, up to 80% of the H1 line can be directed to the retinal progenitor fate, and express a gene expression profile similar to progenitors derived from human fetal retina. The hES cell-derived progenitors differentiate primarily into inner retinal neurons (ganglion and amacrine cells), with functional glutamate receptors. Upon coculture with retinas derived from a mouse model of retinal degeneration, the hES cell derived retinal progenitors integrate with the degenerated mouse retina and increase in their expression of photoreceptor-specific markers. These results demonstrate that human ES cells can be selectively directed to a neural retinal cell fate and thus may be useful in the treatment of retinal degenerations.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

