Probiotics for pediatric antibiotic-associated diarrhea: a meta-analysis of randomized placebo-controlled trials
- PMID: 16908901
- PMCID: PMC1534112
- DOI: 10.1503/cmaj.051603
Probiotics for pediatric antibiotic-associated diarrhea: a meta-analysis of randomized placebo-controlled trials
Erratum in
- CMAJ. 2006 Sep 26;175(7):777
Abstract
Background: Antibiotic treatment is known to disturb gastrointestinal microflora, which results in a range of clinical symptoms--most notably, diarrhea. This is especially important in children, for whom antibiotics are prescribed frequently. Although meta-analyses have been conducted to evaluate the ability of probiotics to prevent antibiotic-induced diarrhea in the general population, little is known about which probiotic strains and doses might be of most benefit to children. Our objective in this study was to assess the efficacy of probiotics (of any specified strain or dose) for the prevention of antibiotic-associated diarrhea in children and to assess adverse events associated with the use of probiotics when coadministered with antibiotics to children.
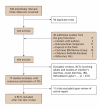
Methods: A comprehensive search was performed of the major electronic databases (e.g., CENTRAL, MEDLINE, EMBASE, CINAHL, AMED) from their inception to January 2005. We also contacted experts and searched registries and meeting abstracts for additional relevant articles. Randomized controlled trials that compared probiotic treatment with placebo or no treatment, involving pediatric subjects less than 19 years of age were included. Two reviewers independently applied eligibility criteria and assessed the studies for methodological quality. Data were independently extracted by 2 reviewers and analyzed via the standard Cochrane methodology.
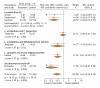
Results: Six studies were included (total n = 707 patients). The combined results, analyzed with a per-protocol method that reported on the incidence of diarrhea during antibiotic treatment, showed significant benefit for the use of probiotics over placebo (relative risk [RR] 0.43, 95% confidence interval [CI] 0.25-0.75, Iota2 = 70.1%). In contrast, results from intention-to-treat analysis were nonsignificant overall (RR 1.01, 95% CI 0.64-1.61). Subgroup analysis on 4 studies that provided at least 5 billion single-strain colony-forming units (CFUs) daily (range 5.5-40 x 10(9) Lactobacillus GG, L. sporogens or Saccharomyces boulardii) showed strong evidence with narrow CIs for the preventative effects of probiotics for antibiotic-associated diarrhea (RR 0.36, 95% CI 0.25-0.53, Iota2 = 3.5%). No serious adverse events were reported.
Interpretation: The potential protective effects of probiotics to prevent antibiotic-associated diarrhea in children do not withstand intention-to-treat analysis. Before routine use is recommended, further studies (with limited losses of subjects to follow-up) are merited. Trials should involve those probiotic strains and doses with the most promising evidence (i.e., Lactobacillus GG, L. sporogens or S. boulardii at 5-40 x 10(9) CFUs daily).
Figures



References
-
- Khaled A, Ahmad F, Brogan T, et al. Prescription medicine use by one million Canadian children. Paediatr Child Health 2003;8(Suppl A):43A-5A.
-
- McFarland LV. Epidemiology, risk factors and treatments for antibiotic-associated diarrhea [review]. Dig Dis 1998;16:292-307. - PubMed
-
- Wistrom J, Norrby SR, Myhre EB, et al. Frequency of antibiotic-associated diarrhoea in 2462 antibiotic-treated hospitalized patients: a prospective study. J Antimicrob Chemother 2001;47:43-50. - PubMed
-
- LaRosa M, Bottaro G, Gulino N, et al. Prevention of antibiotic-associated diarrhea with Lactobacillus sporogens and fructo-oligosaccharides in children: a multi-centric double-blind vs. placebo study. Minerva Pediatr 2003;55:447-52. - PubMed
-
- Turck D, Bernet JP, Marx J, et al. Incidence and risk factors for of oral antibiotic-associated diarrhea in an outpatient pediatric population. J Pediatr Gastroenterol Nutr 2003;37:22-6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
