A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer
- PMID: 16909140
- PMCID: PMC2360678
- DOI: 10.1038/sj.bjc.6603301
A multicenter phase III trial comparing irinotecan-gemcitabine (IG) with gemcitabine (G) monotherapy as first-line treatment in patients with locally advanced or metastatic pancreatic cancer
Abstract
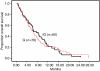
Our purpose was to determine the response rate and median and overall survival of gemcitabine as monotherapy versus gemcitabine plus irinotecan in advanced or metastatic pancreatic cancer. Patients with histologically or cytologically confirmed adenocarcinoma who were chemotherapy and radiotherapy naive were enrolled. Patients were centrally randomised at a one-to-one ratio to receive either gemcitabine monotherapy (900 mg m(-2) on days 1, 8 and 15 every 4 weeks (arm G), or gemcitabine (days 1 and 8) plus irinotecan (300 mg m(-2) on day 8) (arm IG), repeated every 3 weeks. The total number of cycles administered was 255 in the IG arm and 245 in the G arm; the median number of cycles was 3. In all, 145 patients (71 in arm IG and 74 in arm G) were enrolled; 60 and 70 patients from arms IG and G, respectively, were evaluable. A complete clinical response was achieved in three (4.3%) arm G patients; nine (15%) patients in arm IG and four (5.7%) in arm G achieved a partial response. The overall response rate was: arm IG 15% and arm G 10% (95% CI 5.96-24.04 and 95% CI 2.97-17.03, respectively; P=0.387). The median time to tumour progression was 2.8 months and 2.9 months and median survival time was 6.4 and 6.5 months for the IG and G arms, respectively. One-year survival was 24.3% for the IG arm and 21.8% for the G arm. No statistically significant difference was observed comparing gemcitabine monotherapy versus gemcitabine plus irinotecan in the treatment of advanced pancreatic cancer, with respect to overall and 1-year survival.
References
-
- Alberts SR, Townley PM, Goldberg RM, Cha SS, Moore Jr DF, Krook JE, Pilot HC, Fitch TR, Wiesonfeld M, Maillard JA, Sargent DJ (2002) Gemcitabine and oxaliplatin for patients with advanced or pancreatic metastatic cancer: a North Central Cancer Treatment Group (NCCTG) phase I study. Ann Oncol 13: 553–557 - PubMed
-
- Androulakis N, Kouroussis CH, Dimopoulos MA, Samelis G, Kakolyris S, Tsavaris N, Genatas K, Aravantinos G, Papadimitriou CR, Karabekios S, Stathopoulos GP, Georgoulias V (1999) Treatment of pancreatic cancer with docetaxel and granulocyte colony-stimulating factor: a multicenter phase II study. J Clin Oncol 17: 1779–1785 - PubMed
-
- Bahadori HR, Rocha Lima CM, Green MR, Safa AR (1999) Synergistic effect of gemcitabine and irinotecan (CPT-11) on breast and small cell lung cancer cell lines. Anticancer Res 19: 5423–5428 - PubMed
-
- Berlin JD, Catalano P, Thomas JP, Kugler JW, Haller DJ, Benson AB (2002) Phase III study of gemcitabine in combination with fluorouracil versus gemcitabine alone in patients with advanced pancreatic carcinoma. Eastern Cooperative Oncology Group Trial E 2297. J Clin Oncol 20: 3270–3275 - PubMed
-
- Brenna MF, Kinsella TJ, Casper ES (1993) Cancer of the pancreas. In Cancer: Principles and Practice of Oncology DeVita VT Jr Hellman S, Rosenberg SA (eds) 4th edition, pp 849–882. Philadelphia, PA: Lippincott
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical