Stereoselective synthesis of the C(1)-C(13) segment of dolabelide B
- PMID: 16909163
- PMCID: PMC1540402
- DOI: 10.1016/j.tetlet.2005.04.146
Stereoselective synthesis of the C(1)-C(13) segment of dolabelide B
Abstract
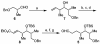
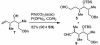
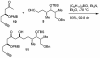
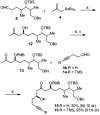
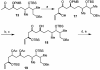
The efficient construction of the C(1)-C(13) segment of dolabelide B is described. A key element of the synthesis entails BITIP catalyzed asymmetric methallylation to establish the C(7) stereocenter, which was then used to direct the stereoselective installation of the C(9) and C(11) centers through Evans reduction and 1,5-anti aldol condensation, respectively.
Figures
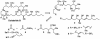
References
-
- Ojika M, Nagoya T, Yamada K. Tetrahedron Lett. 1995;36:7491–7494.
-
- Grimaud L, de Mesmay R, Prunet J. Org. Lett. 2002;4:419–421. - PubMed
-
- Desroy N, Le Roux R, Phansavath P, Chiummiento L, Bonini C, Genêt J-P. Tetrahedron Lett. 2003;44:1763–1766.
-
- Schmidt DR, Park PK, Leighton JL. Org. Lett. 2003;5:3535–3537. - PubMed
-
- Miyaura N, Suzuki A. Chem. Rev. 1995;95:2457–2483.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
