The Forkhead transcription factor Hcm1 regulates chromosome segregation genes and fills the S-phase gap in the transcriptional circuitry of the cell cycle
- PMID: 16912276
- PMCID: PMC1553209
- DOI: 10.1101/gad.1450606
The Forkhead transcription factor Hcm1 regulates chromosome segregation genes and fills the S-phase gap in the transcriptional circuitry of the cell cycle
Abstract
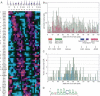
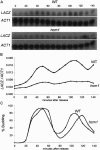
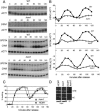
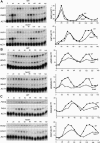
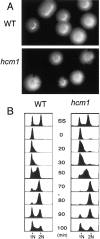
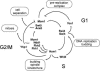
Transcription patterns shift dramatically as cells transit from one phase of the cell cycle to another. To better define this transcriptional circuitry, we collected new microarray data across the cell cycle of budding yeast. The combined analysis of these data with three other cell cycle data sets identifies hundreds of new highly periodic transcripts and provides a weighted average peak time for each transcript. Using these data and phylogenetic comparisons of promoter sequences, we have identified a late S-phase-specific promoter element. This element is the binding site for the forkhead protein Hcm1, which is required for its cell cycle-specific activity. Among the cell cycle-regulated genes that contain conserved Hcm1-binding sites, there is a significant enrichment of genes involved in chromosome segregation, spindle dynamics, and budding. This may explain why Hcm1 mutants show 10-fold elevated rates of chromosome loss and require the spindle checkpoint for viability. Hcm1 also induces the M-phase-specific transcription factors FKH1, FKH2, and NDD1, and two cell cycle-specific transcriptional repressors, WHI5 and YHP1. As such, Hcm1 fills a significant gap in our understanding of the transcriptional circuitry that underlies the cell cycle.
Figures

References
-
- Adams I.R., Kilmartin J.V. Spindle pole body duplication: A model for centrosome duplication? Trends Cell Biol. 2000;10:329–335. - PubMed
-
- Alvarez B., Martinez A.C., Burgering B.M., Carrera A.C. Forkhead transcription factors contribute to execution of the mitotic programme in mammals. Nature. 2001;413:744–747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
