The flagellar-specific transcription factor, sigma28, is the Type III secretion chaperone for the flagellar-specific anti-sigma28 factor FlgM
- PMID: 16912280
- PMCID: PMC1553213
- DOI: 10.1101/gad.380406
The flagellar-specific transcription factor, sigma28, is the Type III secretion chaperone for the flagellar-specific anti-sigma28 factor FlgM
Abstract
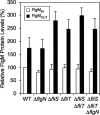
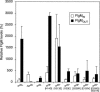
The sigma(28) protein is a member of the bacterial sigma(70)-family of transcription factors that directs RNA polymerase to flagellar late (class 3) promoters. The sigma(28) protein is regulated in response to flagellar assembly by the anti-sigma(28) factor FlgM. FlgM inhibits sigma(28)-dependent transcription of genes whose products are needed late in assembly until the flagellar basal motor structure, the hook-basal body (HBB), is constructed. A second function for the sigma(28) transcription factor has been discovered: sigma(28) facilitates the secretion of FlgM through the HBB, acting as the FlgM Type III secretion chaperone. Transcription-specific mutants in sigma(28) were isolated that remained competent for FlgM-facilitated secretion separating the transcription and secretion-facilitation activities of sigma (28). Conversely, we also describe the isolation of mutants in sigma(28) that are specific for FlgM-facilitated secretion. The data demonstrate that sigma(28) is the Type III secretion chaperone for its own anti-sigma factor FlgM. Thus, a novel role for a sigma(70)-family transcription factor is described.
Figures



References
-
- Akeda Y., Galan J.E. Chaperone release and unfolding of substrates in type III secretion. Nature. 2005;437:911–915. - PubMed
-
- Aldridge P., Hughes K.T. Regulation of flagellar assembly. Curr. Opin. Microbiol. 2002;5:160–165. - PubMed
-
- Aldridge P., Jenal U. Cell cycle-dependent degradation of a flagellar motor component requires a novel-type response regulator. Mol. Microbiol. 1999;32:379–391. - PubMed
-
- Aldridge P., Karlinsey J., Hughes K.T. The type III secretion chaperone FlgN regulates flagellar assembly via a negative feedback loop containing its chaperone substrates FlgK and FlgL. Mol. Microbiol. 2003;49:1333–1345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
