Characterization of a UL49-null mutant: VP22 of herpes simplex virus type 1 facilitates viral spread in cultured cells and the mouse cornea
- PMID: 16912314
- PMCID: PMC1563855
- DOI: 10.1128/JVI.00498-06
Characterization of a UL49-null mutant: VP22 of herpes simplex virus type 1 facilitates viral spread in cultured cells and the mouse cornea
Abstract
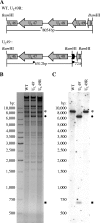
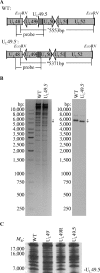
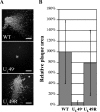
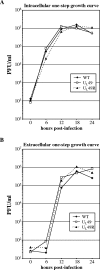
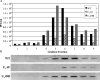
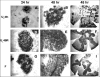
Herpes simplex virus type 1 (HSV-1) virions, like those of all herpesviruses, contain a proteinaceous layer termed the tegument that lies between the nucleocapsid and viral envelope. The HSV-1 tegument is composed of at least 20 different viral proteins of various stoichiometries. VP22, the product of the U(L)49 gene, is one of the most abundant tegument proteins and is conserved among the alphaherpesviruses. Although a number of interesting biological properties have been attributed to VP22, its role in HSV-1 infection is not well understood. In the present study we have generated both a U(L)49-null virus and its genetic repair and characterized their growth in both cultured cells and the mouse cornea. While single-step growth analyses indicated that VP22 is dispensable for virus replication at high multiplicities of infection (MOIs), analyses of plaque morphology and intra- and extracellular multistep growth identified a role for VP22 in viral spread during HSV-1 infection at low MOIs. Specifically, VP22 was not required for either virion infectivity or cell-cell spread but was required for accumulation of extracellular virus to wild-type levels. We found that the absence of VP22 also affected virion composition. Intracellular virions generated by the U(L)49-null virus contained reduced amounts of ICP0 and glycoproteins E and D compared to those generated by the wild-type and U(L)49-repaired viruses. In addition, viral spread in the mouse cornea was significantly reduced upon infection with the U(L)49-null virus compared to infection with the wild-type and U(L)49-repaired viruses, identifying a role for VP22 in viral spread in vivo as well as in vitro.
Figures



Similar articles
-
Assembly of infectious Herpes simplex virus type 1 virions in the absence of full-length VP22.J Virol. 2000 Nov;74(21):10041-54. doi: 10.1128/jvi.74.21.10041-10054.2000. J Virol. 2000. PMID: 11024133 Free PMC article.
-
Bovine Herpesvirus 1 UL49.5 Interacts with gM and VP22 To Ensure Virus Cell-to-Cell Spread and Virion Incorporation: Novel Role for VP22 in gM-Independent UL49.5 Virion Incorporation.J Virol. 2018 Jun 13;92(13):e00240-18. doi: 10.1128/JVI.00240-18. Print 2018 Jul 1. J Virol. 2018. PMID: 29669828 Free PMC article.
-
Functional hierarchy of herpes simplex virus type-1 membrane proteins in corneal infection and virus transmission to ganglionic neurons.Curr Eye Res. 2014 Dec;39(12):1169-77. doi: 10.3109/02713683.2014.906626. Epub 2014 Apr 21. Curr Eye Res. 2014. PMID: 24749493
-
HSV-1 tegument protein and the development of its genome editing technology.Virol J. 2016 Jun 24;13:108. doi: 10.1186/s12985-016-0563-x. Virol J. 2016. PMID: 27343062 Free PMC article. Review.
-
Tegument proteins of human cytomegalovirus.Microbiol Mol Biol Rev. 2008 Jun;72(2):249-65, table of contents. doi: 10.1128/MMBR.00040-07. Microbiol Mol Biol Rev. 2008. PMID: 18535146 Free PMC article. Review.
Cited by
-
Equine herpesvirus type 1 tegument protein VP22 is not essential for pathogenicity in a hamster model, but is required for efficient viral growth in cultured cells.J Vet Med Sci. 2015 Oct;77(10):1293-7. doi: 10.1292/jvms.14-0648. Epub 2015 May 7. J Vet Med Sci. 2015. PMID: 25948053 Free PMC article.
-
A UL47 gene deletion mutant of bovine herpesvirus type 1 exhibits impaired growth in cell culture and lack of virulence in cattle.J Virol. 2010 Jan;84(1):445-58. doi: 10.1128/JVI.01544-09. J Virol. 2010. PMID: 19864376 Free PMC article.
-
Functional interactions between herpes simplex virus pUL51, pUL7 and gE reveal cell-specific mechanisms for epithelial cell-to-cell spread.Virology. 2019 Nov;537:84-96. doi: 10.1016/j.virol.2019.08.014. Epub 2019 Aug 18. Virology. 2019. PMID: 31493658 Free PMC article.
-
The UL14 tegument protein of herpes simplex virus type 1 is required for efficient nuclear transport of the alpha transinducing factor VP16 and viral capsids.J Virol. 2008 Feb;82(3):1094-106. doi: 10.1128/JVI.01226-07. Epub 2007 Nov 21. J Virol. 2008. PMID: 18032514 Free PMC article.
-
Identification of Marek's Disease Virus VP22 Tegument Protein Domains Essential for Virus Cell-to-Cell Spread, Nuclear Localization, Histone Association and Cell-Cycle Arrest.Viruses. 2019 Jun 8;11(6):537. doi: 10.3390/v11060537. Viruses. 2019. PMID: 31181775 Free PMC article.
References
-
- Blaho, J. A., C. Mitchell, and B. Roizman. 1994. An amino acid sequence shared by the herpes simplex virus 1 alpha regulatory proteins 0, 4, 22, and 27 predicts the nucleotidylylation of the UL21, UL31, UL47, and UL49 gene products. J. Biol. Chem. 269:17401-17410. - PubMed

