Interaction between vaccinia virus extracellular virus envelope A33 and B5 glycoproteins
- PMID: 16912323
- PMCID: PMC1563889
- DOI: 10.1128/JVI.00598-06
Interaction between vaccinia virus extracellular virus envelope A33 and B5 glycoproteins
Abstract
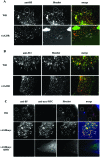
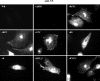
The extracellular form of vaccinia virus acquires its outer envelope by wrapping with cytoplasmic membranes that contain at least seven virus-encoded proteins, of which four are glycoproteins. We searched for interactions between the vaccinia virus A33 glycoprotein and proteins A34, A36, B5, F12, and F13. First, when myc epitope-tagged A33 was expressed in combination with other envelope proteins, A33 colocalized with B5 and A36, suggesting that direct A33-B5 and A33-A36 interactions occur in the absence of infection. A recombinant vaccinia virus (vA33Rmyc) was constructed by introduction of the myc-tagged A33 version (A33myc) into A33-deficient vaccinia virus. A33myc partially restored plaque formation and colocalized with enveloped virions in infected cells. Coimmunoprecipitation experiments with extracts of vA33Rmyc-infected cells confirmed the existence of a physical association of A33 with A36 and B5. Of these, the A33-B5 interaction is a novel finding, whereas the interaction between A33 and A36 has been previously characterized. A collection of vaccinia viruses expressing mutated versions of the B5 protein was used to investigate the domain(s) of B5 required for interaction with A33. Both the cytoplasmic domain and most of the extracellular domain, but not the transmembrane domain, of the B5 protein were dispensable for binding to A33. Mutations in the extracellular portions of B5 and A33 that enhance extracellular virus release did not affect the interaction between the two. In contrast, substituting the B5 transmembrane domain with that of the vesicular stomatitis virus G glycoprotein prevented the association with A33. Immunofluorescence experiments on virus mutants indicated that B5 is required for efficient targeting of A33 into enveloped virions. These results point to the transmembrane domain of B5 as the major determinant of the A33-B5 interaction and demonstrate that protein-protein interactions are crucial in determining the composition of the virus envelope.
Figures







Similar articles
-
Vaccinia Virus Glycoproteins A33, A34, and B5 Form a Complex for Efficient Endoplasmic Reticulum to trans-Golgi Network Transport.J Virol. 2020 Mar 17;94(7):e02155-19. doi: 10.1128/JVI.02155-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31941777 Free PMC article.
-
Vaccinia virus A34 glycoprotein determines the protein composition of the extracellular virus envelope.J Virol. 2008 Mar;82(5):2150-60. doi: 10.1128/JVI.01969-07. Epub 2007 Dec 19. J Virol. 2008. PMID: 18094186 Free PMC article.
-
Role of receptor-mediated endocytosis in the formation of vaccinia virus extracellular enveloped particles.J Virol. 2005 Apr;79(7):4080-9. doi: 10.1128/JVI.79.7.4080-4089.2005. J Virol. 2005. PMID: 15767409 Free PMC article.
-
Viral glycoprotein-mediated cell fusion assays using vaccinia virus vectors.Methods Mol Biol. 2004;269:309-32. doi: 10.1385/1-59259-789-0:309. Methods Mol Biol. 2004. PMID: 15114023 Review.
-
The exit of vaccinia virus from infected cells.Virus Res. 2004 Dec;106(2):189-97. doi: 10.1016/j.virusres.2004.08.015. Virus Res. 2004. PMID: 15567497 Review.
Cited by
-
Exploring monkeypox virus proteins and rapid detection techniques.Front Cell Infect Microbiol. 2024 May 28;14:1414224. doi: 10.3389/fcimb.2024.1414224. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38863833 Free PMC article. Review.
-
Monkeypox virus-infected individuals mount comparable humoral immune responses as Smallpox-vaccinated individuals.Nat Commun. 2023 Sep 23;14(1):5948. doi: 10.1038/s41467-023-41587-x. Nat Commun. 2023. PMID: 37741831 Free PMC article.
-
There is an A33-dependent mechanism for the incorporation of B5-GFP into vaccinia virus extracellular enveloped virions.Virology. 2010 Jun 20;402(1):83-93. doi: 10.1016/j.virol.2010.03.017. Epub 2010 Apr 7. Virology. 2010. PMID: 20378144 Free PMC article.
-
The Ectodomain of the Vaccinia Virus Glycoprotein A34 Is Required for Cell Binding by Extracellular Virions and Contains a Large Region Capable of Interaction with Glycoprotein B5.J Virol. 2019 Feb 5;93(4):e01343-18. doi: 10.1128/JVI.01343-18. Print 2019 Feb 15. J Virol. 2019. PMID: 30463966 Free PMC article.
-
Vaccinia Virus Glycoproteins A33, A34, and B5 Form a Complex for Efficient Endoplasmic Reticulum to trans-Golgi Network Transport.J Virol. 2020 Mar 17;94(7):e02155-19. doi: 10.1128/JVI.02155-19. Print 2020 Mar 17. J Virol. 2020. PMID: 31941777 Free PMC article.
References
-
- Blasco, R., and B. Moss. 1995. Selection of recombinant vaccinia viruses on the basis of plaque formation. Gene 158:157-162. - PubMed
-
- Borrego, B., M. M. Lorenzo, and R. Blasco. 1999. Complementation of P37 (F13L gene) knock-out in vaccinia virus by a cell line expressing the gene constitutively. J. Gen. Virol. 80:425-432. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

