Invasion of host cells by Salmonella typhimurium requires focal adhesion kinase and p130Cas
- PMID: 16914515
- PMCID: PMC1635395
- DOI: 10.1091/mbc.e06-06-0492
Invasion of host cells by Salmonella typhimurium requires focal adhesion kinase and p130Cas
Abstract
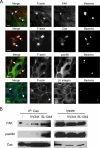
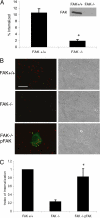
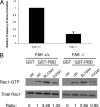
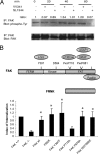
Salmonella typhimurium colonizes the intestinal epithelium by injecting an array of effector proteins into host cells that induces phagocytic uptake of attached bacteria. However, the host molecules targeted by these effectors remain poorly defined. Here, we demonstrate that S. typhimurium induces formation of focal adhesion-like complexes at sites of bacterial attachment and that both focal adhesion kinase (FAK) and the scaffolding protein p130Cas are required for Salmonella uptake. Entry of Salmonella into FAK(-/-) cells is dramatically impaired and can be restored to control levels by expression of wild-type FAK. Surprisingly, reconstitution of bacterial internalization requires neither the kinase domain of FAK nor activation of c-Src, but does require a C-terminal PXXP motif through which FAK interacts with Cas. Infection of Cas(-/-) cells is also impaired, and reconstitution of invasiveness requires the central Cas YXXP repeat domain. The invasion defect in Cas(-/-) cells can be suppressed by overexpression of FAK, suggesting a functional link between FAK and Cas in the regulation of Salmonella invasion. Together, these findings reveal a novel role for focal adhesion proteins in the invasion of host cells by Salmonella.
Figures




References
-
- Agerer F., Lux S., Michel A., Rohde M., Ohlsen K., Hauck C. R. Cellular invasion by Staphylococcus aureus reveals a functional link between focal adhesion kinase and cortactin in integrin-mediated internalisation. J. Cell Sci. 2005;118:2189–2200. - PubMed
-
- Astier A., Avraham H., Manie S. N., Groopman J., Canty T., Avraham S., Freedman A. S. The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after beta1-integrin stimulation in B cells and binds to p130cas. J. Biol. Chem. 1997;272:228–232. - PubMed
-
- Bouton A. H., Riggins R. B., Bruce-Staskal P. J. Functions of the adapter protein Cas: signal convergence and the determination of cellular responses. Oncogene. 2001;20:6448–6458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

