Gene targeting of Cdc42 and Cdc42GAP affirms the critical involvement of Cdc42 in filopodia induction, directed migration, and proliferation in primary mouse embryonic fibroblasts
- PMID: 16914516
- PMCID: PMC1635400
- DOI: 10.1091/mbc.e06-05-0466
Gene targeting of Cdc42 and Cdc42GAP affirms the critical involvement of Cdc42 in filopodia induction, directed migration, and proliferation in primary mouse embryonic fibroblasts
Abstract
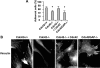
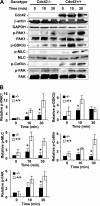
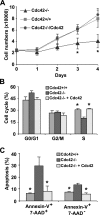
Recent studies in Cdc42 knockout mouse embryonic stem (ES) cells and ES-derived fibroblastoid cell lines raise concern on a body of literature derived by dominant mutant expression approach in a variety of cell lines implicating mammalian Cdc42 as a key regulator of filopodia induction, directional migration and cell cycle progression. To resolve the physiological function of mammalian Cdc42, we have characterized the Cdc42(-/-) and Cdc42GAP(-/-) primary mouse embryonic fibroblasts (MEFs) produced by gene targeting as the Cdc42 loss- or gain-of-activity cell model. The Cdc42(-/-) cells were defective in filopodia formation stimulated by bradykinin and in dorsal membrane ruffling stimulated by PDGF, whereas the Cdc42GAP(-/-) cells displayed spontaneous filopodia. The Cdc42 loss- or gain-of-activity cells were defective in adhesion to fibronectin, wound-healing, polarity establishment, and migration toward a serum gradient. These defects were associated with deficiencies of PAK1, GSK3beta, myosin light chain, and FAK phosphorylation. Furthermore, Cdc42(-/-) cells were defective in G1/S-phase transition and survival, correlating with deficient NF-kappaB transcription and defective JNK, p70 S6K, and ERK1/2 activation. These results demonstrate a different requirement of Cdc42 activity in primary MEFs from ES or ES-derived clonal fibroblastoid cells and suggest that Cdc42 plays cell-type-specific signaling roles.
Figures




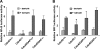

References
-
- Chen F., et al. Cdc42 is required for PIP(2)-induced actin polymerization and early development but not for cell viability. Curr. Biol. 2000;10:758–765. - PubMed
-
- Chou M. M., Masuda-Robens J. M., Gupta M. L. Cdc42 promotes G1 progression through p70 S6 kinase-mediated induction of cyclin E expression. J. Biol. Chem. 2003;278:35241–35247. - PubMed
-
- Debidda M., Wang L., Zang H., Poli V., Zheng Y. A role of STAT3 in Rho GTPase-regulated cell migration and proliferation. J. Biol. Chem. 2005;280:17275–17285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

