Differential effects of NMDA and AMPA glutamate receptors on functional magnetic resonance imaging signals and evoked neuronal activity during forepaw stimulation of the rat
- PMID: 16914666
- PMCID: PMC6674350
- DOI: 10.1523/JNEUROSCI.4615-05.2006
Differential effects of NMDA and AMPA glutamate receptors on functional magnetic resonance imaging signals and evoked neuronal activity during forepaw stimulation of the rat
Abstract
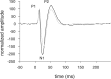
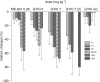
Most of the currently used methods for functional brain imaging do not visualize neuronal activity directly but rather rely on the elicited hemodynamic and/or metabolic responses. Glutamate, the major excitatory neurotransmitter, plays an important role in the neurovascular/neurometabolic coupling, but the specific mechanisms are still poorly understood. To investigate the role of the two major ionotropic glutamate receptors [NMDA receptors (NMDA-Rs) and AMPA receptors (AMPA-Rs)] for the generation of functional magnetic resonance imaging (fMRI) signals, we used fMRI [measurements of blood oxygenation level-dependent (BOLD), perfusion-weighted imaging (PWI), and cerebral blood volume (CBV)] together with recordings of somatosensory evoked potentials (SEPs) during electrical forepaw stimulation in the alpha-chloralose anesthetized rat. Intravenous injection of the NMDA-R antagonist MK-801 [(+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine maleate] (0.06 mg/kg plus 3.6 microg x kg(-1) x h(-1)) significantly decreased BOLD (-51 +/- 19%; n = 5) and PWI (-57 +/- 26%; n = 5) responses but reduced the SEPs only mildly (approximately -10%). Systemic application of the AMPA-R antagonist GYKI-53655 [1-(4-aminophenyl)-3-methylcarbamyl-4-methyl-7,8-methylenedioxy-3,4-dihydro-5H-2,3-benzodiazepine] significantly decreased both the hemodynamic response (BOLD, -49 +/- 13 and -65 +/- 15%; PWI, -22 +/- 48 and -68 +/- 4% for 5 and 7 mg/kg, i.v., respectively; CBV, -80 +/- 7% for 7 mg/kg; n = 4) and the SEPs (up to -60%). These data indicate that the interaction of glutamate with its postsynaptic and/or glial receptors is necessary for the generation of blood flow and BOLD responses and illustrate the differential role of NMDA-Rs and AMPA-Rs in the signaling chain leading from increased neuronal activity to the hemodynamic response in the somatosensory cortex.
Figures



Similar articles
-
Characterization of the glutamate receptors mediating release of somatostatin from cultured hippocampal neurons.J Neurochem. 1996 Jan;66(1):161-8. doi: 10.1046/j.1471-4159.1996.66010161.x. J Neurochem. 1996. PMID: 8522949
-
Nonlinear neurovascular coupling in rat sensory cortex by activation of transcallosal fibers.J Cereb Blood Flow Metab. 2007 Mar;27(3):575-87. doi: 10.1038/sj.jcbfm.9600372. Epub 2006 Aug 9. J Cereb Blood Flow Metab. 2007. PMID: 16896350
-
Postsynaptic activity of inhibitory neurons evokes hemodynamic fMRI responses.Neuroimage. 2021 Jan 15;225:117457. doi: 10.1016/j.neuroimage.2020.117457. Epub 2020 Oct 16. Neuroimage. 2021. PMID: 33069862 Free PMC article.
-
Contribution of AMPA and NMDA receptors to excitatory responses in the inferior colliculus.Hear Res. 2002 Jun;168(1-2):35-42. doi: 10.1016/s0378-5955(02)00372-6. Hear Res. 2002. PMID: 12117507 Review.
-
Separating vascular and neuronal effects of age on fMRI BOLD signals.Philos Trans R Soc Lond B Biol Sci. 2021 Jan 4;376(1815):20190631. doi: 10.1098/rstb.2019.0631. Epub 2020 Nov 16. Philos Trans R Soc Lond B Biol Sci. 2021. PMID: 33190597 Free PMC article. Review.
Cited by
-
Intrinsic functional architecture of the non-human primate spinal cord derived from fMRI and electrophysiology.Nat Commun. 2019 Mar 29;10(1):1416. doi: 10.1038/s41467-019-09485-3. Nat Commun. 2019. PMID: 30926817 Free PMC article.
-
Characterization of NO-producing neurons in the rat corpus callosum.Brain Behav. 2014 May;4(3):317-36. doi: 10.1002/brb3.218. Epub 2014 Feb 12. Brain Behav. 2014. PMID: 24944862 Free PMC article.
-
Cortical plasticity is associated with blood-brain barrier modulation.Elife. 2024 Jul 18;12:RP89611. doi: 10.7554/eLife.89611. Elife. 2024. PMID: 39024007 Free PMC article.
-
No increase of the blood oxygenation level-dependent functional magnetic resonance imaging signal with higher field strength: implications for brain activation studies.J Neurosci. 2010 Apr 14;30(15):5234-41. doi: 10.1523/JNEUROSCI.0844-10.2010. J Neurosci. 2010. PMID: 20392946 Free PMC article.
-
Key role of tissue plasminogen activator in neurovascular coupling.Proc Natl Acad Sci U S A. 2008 Jan 22;105(3):1073-8. doi: 10.1073/pnas.0708823105. Epub 2008 Jan 14. Proc Natl Acad Sci U S A. 2008. PMID: 18195371 Free PMC article.
References
-
- Ahissar E, Sosnik R, Haidarliu S (2000). Transformation from temporal to rate coding in a somatosensory thalamocortical pathway. Nature 406:302–306. - PubMed
-
- Ances BM, Greenberg JH, Detre JA (2000a). Effects of variations in interstimulus interval on activation-flow coupling response and somatosensory evoked potentials with forepaw stimulation in the rat. J Cereb Blood Flow Metab 20:290–297. - PubMed
-
- Ances BM, Zarahn E, Greenberg JH, Detre JA (2000b). Coupling of neural activation to blood flow in the somatosensory cortex of rats is time-intensity separable, but not linear. J Cereb Blood Flow Metab 20:921–930. - PubMed
-
- Attwell D, Iadecola C (2002). The neural basis of functional brain imaging signals. Trends Neurosci 25:621–625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
