Corticofugal output from the primary somatosensory cortex selectively modulates innocuous and noxious inputs in the rat spinothalamic system
- PMID: 16914669
- PMCID: PMC6674349
- DOI: 10.1523/JNEUROSCI.1293-06.2006
Corticofugal output from the primary somatosensory cortex selectively modulates innocuous and noxious inputs in the rat spinothalamic system
Abstract
Sensory maps for pain can be modified by deafferentation or injury, and such plasticity has been attributed mainly to changes in the convergence of projections in "bottom-up" mechanisms. We addressed the possible contribution of "top-down" mechanisms by investigating the functional significance of corticofugal influences from the primary somatosensory cortex (S1) to the ventroposterolateral thalamic nucleus (VPL). The strong convergence of spinal and lemniscal afferents to the VPL and the close correspondence between afferents and efferents within the VPL-S1 network suggest the existence of functionally related thalamocortical circuits that are implicated in the detection of innocuous and noxious inputs. Functional characterization of single nociceptive, wide dynamic range, and non-nociceptive VPL neurons and labeling the axons and terminal fields with the juxtacellular technique showed that all three types of cells project to a restricted area, within S1. The convergence of the terminal trees of axons from VPL neurons activated by innocuous, noxious, or both inputs suggests that their inputs are not segregated into anatomically distinct regions. Microinjections within S1 were performed for pharmacological manipulation of corticofugal modulation. Glutamatergic activation of corticofugal output enhanced noxious-evoked responses and affected in a biphasic way tactile-evoked responses of VPL cells. GABA(A)-mediated depression of corticofugal output concomitantly depressed noxious and enhanced innocuous-evoked responses of VPL neurons. Microinjections of a GABA(A) antagonist on corticofugal cells enhanced noxious-evoked responses of VPL cells. Our findings demonstrate that corticofugal influences from S1 contribute to selectively modulate somatosensory submodalities at the thalamic level.
Figures

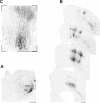
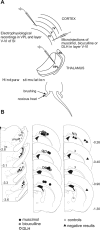
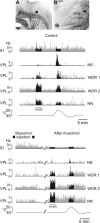
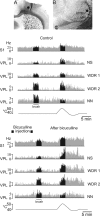
Similar articles
-
Corticofugal outputs facilitate acute, but inhibit chronic pain in rats.Pain. 2009 Mar;142(1-2):108-15. doi: 10.1016/j.pain.2008.12.016. Epub 2009 Jan 23. Pain. 2009. PMID: 19167812
-
Dorsal column-thalamic pathway is involved in thalamic hyperexcitability following peripheral nerve injury: a lesion study in rats with experimental mononeuropathy.Pain. 2000 Mar;85(1-2):263-71. doi: 10.1016/s0304-3959(99)00279-1. Pain. 2000. PMID: 10692627
-
Inhibitory control of nociceptive responses of trigeminal spinal nucleus cells by somatosensory corticofugal projection in rat.Neuroscience. 2012 Sep 27;221:115-24. doi: 10.1016/j.neuroscience.2012.07.003. Epub 2012 Jul 13. Neuroscience. 2012. PMID: 22796078
-
Pain pathways in the primate.Prog Clin Biol Res. 1985;176:117-33. Prog Clin Biol Res. 1985. PMID: 3923492 Review.
-
[Neurophysiologic mechanisms of pain].Fiziol Zh (1978). 1980 Mar-Apr;26(2):235-44. Fiziol Zh (1978). 1980. PMID: 6153993 Review. Russian. No abstract available.
Cited by
-
Distribution and properties of visceral nociceptive neurons in rabbit cingulate cortex.Pain. 2008 Mar;135(1-2):160-74. doi: 10.1016/j.pain.2007.09.024. Epub 2007 Nov 19. Pain. 2008. PMID: 18022321 Free PMC article.
-
Cortical astrocytes rewire somatosensory cortical circuits for peripheral neuropathic pain.J Clin Invest. 2016 May 2;126(5):1983-97. doi: 10.1172/JCI82859. Epub 2016 Apr 11. J Clin Invest. 2016. PMID: 27064281 Free PMC article.
-
Corticofugal projections induce long-lasting effects on somatosensory responses in the trigeminal complex of the rat.Front Syst Neurosci. 2014 May 22;8:100. doi: 10.3389/fnsys.2014.00100. eCollection 2014. Front Syst Neurosci. 2014. PMID: 24904321 Free PMC article.
-
Neuroendocrine pathway involvement in the loss of the cutaneous pressure-induced vasodilatation during acute pain in rats.J Physiol. 2007 Feb 15;579(Pt 1):247-54. doi: 10.1113/jphysiol.2006.121426. Epub 2006 Dec 7. J Physiol. 2007. PMID: 17158176 Free PMC article.
-
Primary somatosensory cortex bidirectionally modulates sensory gain and nociceptive behavior in a layer-specific manner.Nat Commun. 2023 May 24;14(1):2999. doi: 10.1038/s41467-023-38798-7. Nat Commun. 2023. PMID: 37225702 Free PMC article.
References
-
- Albe-Fessard D, Berkley KJ, Kruger L, Ralston HJ, Willis WD (1985). Diencephalic mechanisms of pain sensation. Brain Res Rev 9:217–296. - PubMed
-
- Apkarian AV, Stea RA, Manglos SH, Szeverenyi NM, King RB, Thomas FD (1992). Persistent pain inhibits contralateral somatosensory cortical activity in humans. Neurosci Lett 140:141–147. - PubMed
-
- Apkarian AV, Stea RA, Bolanowski SJ (1994). Heat-induced pain diminishes vibrotactile perception: a touch gate. Somatosens Mot Res 11:259–267. - PubMed
-
- Chmielowska J, Carvell GE, Simons DJ (1989). Spatial organization of thalamocortical and corticothalamic projection systems in the rat SmI barrel cortex. J Comp Neurol 285:325–338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
