Exchange protein activated by cyclic AMP (Epac)-mediated induction of suppressor of cytokine signaling 3 (SOCS-3) in vascular endothelial cells
- PMID: 16914720
- PMCID: PMC1592846
- DOI: 10.1128/MCB.00207-06
Exchange protein activated by cyclic AMP (Epac)-mediated induction of suppressor of cytokine signaling 3 (SOCS-3) in vascular endothelial cells
Abstract
Here, we demonstrate that elevation of intracellular cyclic AMP (cAMP) in vascular endothelial cells (ECs) by either a direct activator of adenylyl cyclase or endogenous cAMP-mobilizing G protein-coupled receptors inhibited the tyrosine phosphorylation of STAT proteins by an interleukin 6 (IL-6) receptor trans-signaling complex (soluble IL-6Ralpha/IL-6). This was associated with the induction of suppressor of cytokine signaling 3 (SOCS-3), a bona fide inhibitor in vivo of gp130, the signal-transducing component of the IL-6 receptor complex. Attenuation of SOCS-3 induction in either ECs or SOCS-3-null murine embryonic fibroblasts abolished the inhibitory effect of cAMP, whereas inhibition of SHP-2, another negative regulator of gp130, was without effect. Interestingly, the inhibition of STAT phosphorylation and SOCS-3 induction did not require cAMP-dependent protein kinase activity but could be recapitulated upon selective activation of the alternative cAMP sensor Epac, a guanine nucleotide exchange factor for Rap1. Consistent with this hypothesis, small interfering RNA-mediated knockdown of Epac1 was sufficient to attenuate both cAMP-mediated SOCS-3 induction and inhibition of STAT phosphorylation, suggesting that Epac activation is both necessary and sufficient to observe these effects. Together, these data argue for the existence of a novel cAMP/Epac/Rap1/SOCS-3 pathway for limiting IL-6 receptor signaling in ECs and illuminate a new mechanism by which cAMP may mediate its potent anti-inflammatory effects.
Figures

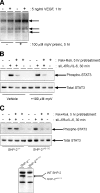


 , P < 0.05 versus the response observed with sIL-6Rα/IL-6 alone). (C) HAECs were pretreated for 5 h with 10 μM Fsk prior to the preparation of soluble cell extracts. Following equalization for protein content, samples were fractionated by SDS-PAGE for immunoblotting with anti-SOCS-3 and anti-GAPDH antibodies, with the latter serving as a loading control.
, P < 0.05 versus the response observed with sIL-6Rα/IL-6 alone). (C) HAECs were pretreated for 5 h with 10 μM Fsk prior to the preparation of soluble cell extracts. Following equalization for protein content, samples were fractionated by SDS-PAGE for immunoblotting with anti-SOCS-3 and anti-GAPDH antibodies, with the latter serving as a loading control.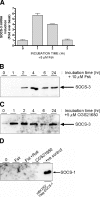

 , P < 0.05 versus the response observed with vector plus MG132). (D) HUVECs were pretreated for 5 h with or without 50 μM 8-pCPT prior to the addition of vehicle, 2.5 ng/ml sIL-6Rα, and 0.5 ng/ml IL-6 for a further 30 min as indicated. Soluble cell extracts equalized for protein content were then fractionated by SDS-PAGE for immunoblotting with anti-Tyr705 phospho-STAT3 and total STAT3 antibodies. (E) HUVECs were transfected twice over 48 h with nontargeting control and Epac1-specific siRNAs prior to treatment with Fsk for 5 h as indicated. Soluble cell extracts equalized for protein content were then fractionated by SDS-PAGE for immunoblotting with anti-Epac1, SOCS-3, and GAPDH antibodies. (F) HUVECs were transfected with control and Epac1-specific siRNAs as described for panel E prior to pretreatment with or without Fsk+Roli for 5 h followed by exposure to 25 ng/ml sIL-6Rα and 5 ng/ml IL-6 for 30 min as indicated. Soluble cell extracts equalized for protein content were then fractionated by SDS-PAGE for immunoblotting with anti-Tyr705 phospho-STAT3 and total STAT3 antibodies.
, P < 0.05 versus the response observed with vector plus MG132). (D) HUVECs were pretreated for 5 h with or without 50 μM 8-pCPT prior to the addition of vehicle, 2.5 ng/ml sIL-6Rα, and 0.5 ng/ml IL-6 for a further 30 min as indicated. Soluble cell extracts equalized for protein content were then fractionated by SDS-PAGE for immunoblotting with anti-Tyr705 phospho-STAT3 and total STAT3 antibodies. (E) HUVECs were transfected twice over 48 h with nontargeting control and Epac1-specific siRNAs prior to treatment with Fsk for 5 h as indicated. Soluble cell extracts equalized for protein content were then fractionated by SDS-PAGE for immunoblotting with anti-Epac1, SOCS-3, and GAPDH antibodies. (F) HUVECs were transfected with control and Epac1-specific siRNAs as described for panel E prior to pretreatment with or without Fsk+Roli for 5 h followed by exposure to 25 ng/ml sIL-6Rα and 5 ng/ml IL-6 for 30 min as indicated. Soluble cell extracts equalized for protein content were then fractionated by SDS-PAGE for immunoblotting with anti-Tyr705 phospho-STAT3 and total STAT3 antibodies.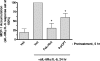
 , P < 0.05 versus sIL-6Rα/IL-6-stimulated MCP-1 levels). Basal MCP-1 levels were 0.69 ± 0.42 ng/ml (n = 3).
, P < 0.05 versus sIL-6Rα/IL-6-stimulated MCP-1 levels). Basal MCP-1 levels were 0.69 ± 0.42 ng/ml (n = 3).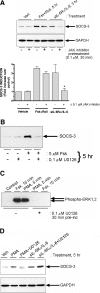
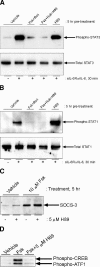
 , P < 0.05 versus sIL-6Rα/IL-6-stimulated SOCS-3 expression in the absence of JAK inhibitor). (B) HUVECs were pretreated for 30 min with or without MEK inhibitor U0126 (0.1 μM) prior to the addition of 5 μM Fsk for a further 5 h as indicated. Soluble cell extracts equalized for protein content were then fractionated by SDS-PAGE for immunoblotting with anti-SOCS-3 antibody. (C) HUVECs were pretreated with or without 0.1 μM U0126 prior to the addition of 5 μM Fsk or 1 μM PMA as indicated. Soluble cell extracts equalized for protein content were then fractionated by SDS-PAGE for immunoblotting with anti-Thr202/Tyr204 phospho-ERK1/2 antibody. (D) HUVECs were pretreated with or without 0.1 μM U0126 prior to the addition of 1 μM PMA or 25 ng/ml sIL-6Rα and 5 ng/ml IL-6 for 5 h as indicated. Soluble cell extracts equalized for protein content were then fractionated by SDS-PAGE for immunoblotting with anti-SOCS-3 and GAPDH antibodies. Veh, vehicle; pre-inc, preincubation.
, P < 0.05 versus sIL-6Rα/IL-6-stimulated SOCS-3 expression in the absence of JAK inhibitor). (B) HUVECs were pretreated for 30 min with or without MEK inhibitor U0126 (0.1 μM) prior to the addition of 5 μM Fsk for a further 5 h as indicated. Soluble cell extracts equalized for protein content were then fractionated by SDS-PAGE for immunoblotting with anti-SOCS-3 antibody. (C) HUVECs were pretreated with or without 0.1 μM U0126 prior to the addition of 5 μM Fsk or 1 μM PMA as indicated. Soluble cell extracts equalized for protein content were then fractionated by SDS-PAGE for immunoblotting with anti-Thr202/Tyr204 phospho-ERK1/2 antibody. (D) HUVECs were pretreated with or without 0.1 μM U0126 prior to the addition of 1 μM PMA or 25 ng/ml sIL-6Rα and 5 ng/ml IL-6 for 5 h as indicated. Soluble cell extracts equalized for protein content were then fractionated by SDS-PAGE for immunoblotting with anti-SOCS-3 and GAPDH antibodies. Veh, vehicle; pre-inc, preincubation.References
-
- Aird, W. C. 2005. Spatial and temporal dynamics of the endothelium. J. Thromb. Haemost. 3:1392-1406. - PubMed
-
- Alexander, W. S., and D. J. Hilton. 2004. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu. Rev. Immunol. 22:503-529. - PubMed
-
- Blanchard, F., W. Yanping, E. Kinzie, L. Duplomb, A. Godard, and H. Baumann. 2001. Oncostatin M regulates the synthesis and turnover of gp130, leukemia inhibitory factor receptor α, and oncostatin M receptor-β by distinct mechanisms. J. Biol. Chem. 276:47038-47045. - PubMed
-
- Bos, J. L. 2003. Epac: a new cAMP target and new avenues in cAMP research. Nat. Rev. Mol. Cell Biol. 4:733-738. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
