Mice with a targeted mutation of patched2 are viable but develop alopecia and epidermal hyperplasia
- PMID: 16914743
- PMCID: PMC1592833
- DOI: 10.1128/MCB.00295-06
Mice with a targeted mutation of patched2 are viable but develop alopecia and epidermal hyperplasia
Abstract
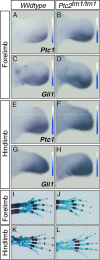
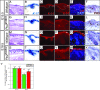
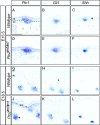
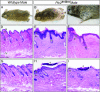
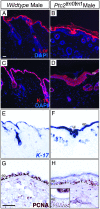
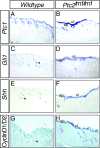
Hedgehog (Hh) signaling plays pivotal roles in tissue patterning and development in Drosophila melanogaster and vertebrates. The Patched1 (Ptc1) gene, encoding the Hh receptor, is mutated in nevoid basal cell carcinoma syndrome, a human genetic disorder associated with developmental abnormalities and increased incidences of basal cell carcinoma (BCC) and medulloblastoma (MB). Ptc1 mutations also occur in sporadic forms of BCC and MB. Mutational studies with mice have verified that Ptc1 is a tumor suppressor. We previously identified a second mammalian Patched gene, Ptc2, and demonstrated its distinct expression pattern during embryogenesis, suggesting a unique role in development. Most notably, Ptc2 is expressed in an overlapping pattern with Shh in the epidermal compartment of developing hair follicles and is highly expressed in the developing limb bud, cerebellum, and testis. Here, we describe the generation and phenotypic analysis of Ptc2(tm1/tm1) mice. Our molecular analysis suggests that Ptc2(tm1) likely represents a hypomorphic allele. Despite the dynamic expression of Ptc2 during embryogenesis, Ptc2(tm1/tm1) mice are viable, fertile, and apparently normal. Interestingly, adult Ptc2(tm1/tm1) male animals develop skin lesions consisting of alopecia, ulceration, and epidermal hyperplasia. While functional compensation by Ptc1 might account for the lack of a strong mutant phenotype in Ptc2-deficient mice, our results suggest that normal Ptc2 function is required for adult skin homeostasis.
Figures




References
-
- Adolphe, C., M. Narang, T. Ellis, C. Wicking, P. Kaur, and B. Wainwright. 2004. An in vivo comparative study of sonic, desert and Indian hedgehog reveals that hedgehog pathway activity regulates epidermal stem cell homeostasis. Development 131:5009-5019. - PubMed
-
- Ashcroft, G. S., M. A. Horan, and M. W. Ferguson. 1998. Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab Investig. 78:47-58. - PubMed
-
- Aszterbaum, M., A. Rothman, R. L. Johnson, M. Fisher, J. Xie, J. M. Bonifas, X. Zhang, M. P. Scott, and E. H. Epstein, Jr. 1998. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J. Investig. Dermatol. 110:885-888. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
