From fate to function: the Drosophila trachea and salivary gland as models for tubulogenesis
- PMID: 16916373
- PMCID: PMC2827874
- DOI: 10.1111/j.1432-0436.2006.00095.x
From fate to function: the Drosophila trachea and salivary gland as models for tubulogenesis
Abstract
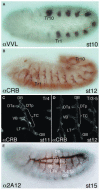
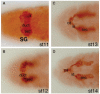
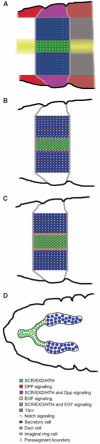
Tube formation is a ubiquitous process required to sustain life in multicellular organisms. The tubular organs of adult mammals include the lungs, vasculature, digestive and excretory systems, as well as secretory organs such as the pancreas, salivary, prostate, and mammary glands. Other tissues, including the embryonic heart and neural tube, have requisite stages of tubular organization early in development. To learn the molecular and cellular basis of how epithelial cells are organized into tubular organs of various shapes and sizes, investigators have focused on the Drosophila trachea and salivary gland as model genetic systems for branched and unbranched tubes, respectively. Both organs begin as polarized epithelial placodes, which through coordinated cell shape changes, cell rearrangement, and cell migration form elongated tubes. Here, we discuss what has been discovered regarding the details of cell fate specification and tube formation in the two organs; these discoveries reveal significant conservation in the cellular and molecular events of tubulogenesis.
Figures

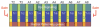





References
-
- Abrams EW, Andrew DJ. Prolyl 4-hydroxylase alpha-related proteins in Drosophila melanogaster: tissue-specific embryonic expression of the 99F8-9 cluster. Mech Dev. 2002;112:165–171. - PubMed
-
- Abrams EW, Andrew DJ. CrebA regulates secretory activity in the Drosophila salivary gland and epidermis. Development. 2005;132:2743–2758. - PubMed
-
- Abrams EW, Mihoulides WK, Andrew DJ. Fork head and Sage maintain a uniform and patent salivary gland lumen through regulation of two downstream target genes, PH4αSG1 and PH4αSG2. Development. 2006 in press. - PubMed
-
- Abrams EW, Vining MS, Andrew DJ. Constructing an organ: the Drosophila salivary gland as a model for tube formation. Trends Cell Biol. 2003;13:247–254. - PubMed
-
- Affolter M, Montagne J, Walldorf U, Groppe J, Kloter U, LaRosa M, Gehring WJ. The Drosophila SRF homolog is expressed in a subset of tracheal cells and maps within a genomic region required for tracheal development. Development. 1994b;120:743–753. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

