ABCG2 expression, function, and promoter methylation in human multiple myeloma
- PMID: 16917002
- PMCID: PMC1895461
- DOI: 10.1182/blood-2005-10-009084
ABCG2 expression, function, and promoter methylation in human multiple myeloma
Abstract
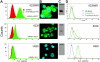
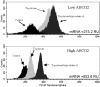
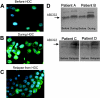
We investigated the role of the breast cancer resistance protein (BCRP/ABCG2) in drug resistance in multiple myeloma (MM). Human MM cell lines, and MM patient plasma cells isolated from bone marrow, were evaluated for ABCG2 mRNA expression by quantitative polymerase chain reaction (PCR) and ABCG2 protein, by Western blot analysis, immunofluorescence microscopy, and flow cytometry. ABCG2 function was determined by measuring topotecan and doxorubicin efflux using flow cytometry, in the presence and absence of the specific ABCG2 inhibitor, tryprostatin A. The methylation of the ABCG2 promoter was determined using bisulfite sequencing. We found that ABCG2 expression in myeloma cell lines increased after exposure to topotecan and doxorubicin, and was greater in logphase cells when compared with quiescent cells. Myeloma patients treated with topotecan had an increase in ABCG2 mRNA and protein expression after treatment with topotecan, and at relapse. Expression of ABCG2 is regulated, at least in part, by promoter methylation both in cell lines and in patient plasma cells. Demethylation of the promoter increased ABCG2 mRNA and protein expression. These findings suggest that ABCG2 is expressed and functional in human myeloma cells, regulated by promoter methylation, affected by cell density, up-regulated in response to chemotherapy, and may contribute to intrinsic drug resistance.
Figures




Similar articles
-
Expression and functional analyses of breast cancer resistance protein in lung cancer.Clin Cancer Res. 2003 Aug 1;9(8):3052-7. Clin Cancer Res. 2003. PMID: 12912956
-
Chemotherapeutic drug-induced ABCG2 promoter demethylation as a novel mechanism of acquired multidrug resistance.Neoplasia. 2009 Dec;11(12):1359-70. doi: 10.1593/neo.91314. Neoplasia. 2009. PMID: 20019844 Free PMC article.
-
Melatonin-induced methylation of the ABCG2/BCRP promoter as a novel mechanism to overcome multidrug resistance in brain tumour stem cells.Br J Cancer. 2013 May 28;108(10):2005-12. doi: 10.1038/bjc.2013.188. Epub 2013 Apr 30. Br J Cancer. 2013. PMID: 23632480 Free PMC article.
-
Multidrug resistance in cancer chemotherapy and xenobiotic protection mediated by the half ATP-binding cassette transporter ABCG2.Curr Med Chem Anticancer Agents. 2004 Jan;4(1):31-42. doi: 10.2174/1568011043482205. Curr Med Chem Anticancer Agents. 2004. PMID: 14754410 Review.
-
Up-regulation of multidrug resistance protein MDR1/ABCB1 in carfilzomib-resistant multiple myeloma differentially affects efficacy of anti-myeloma drugs.Leuk Res. 2021 Feb;101:106499. doi: 10.1016/j.leukres.2020.106499. Epub 2020 Dec 31. Leuk Res. 2021. PMID: 33422770 Review. No abstract available.
Cited by
-
Association between DNA methylation in the miR-328 5'-flanking region and inter-individual differences in miR-328 and BCRP expression in human placenta.PLoS One. 2013 Aug 21;8(8):e72906. doi: 10.1371/journal.pone.0072906. eCollection 2013. PLoS One. 2013. PMID: 23991164 Free PMC article.
-
The ABCG family of membrane-associated transporters: you don't have to be big to be mighty.Br J Pharmacol. 2011 Dec;164(7):1767-79. doi: 10.1111/j.1476-5381.2010.01177.x. Br J Pharmacol. 2011. PMID: 21175590 Free PMC article. Review.
-
Chemotherapy curable malignancies and cancer stem cells: a biological review and hypothesis.BMC Cancer. 2016 Nov 21;16(1):906. doi: 10.1186/s12885-016-2956-z. BMC Cancer. 2016. PMID: 27871274 Free PMC article. Review.
-
MicroRNA-212/ABCG2-axis contributes to development of imatinib-resistance in leukemic cells.Oncotarget. 2017 Sep 26;8(54):92018-92031. doi: 10.18632/oncotarget.21272. eCollection 2017 Nov 3. Oncotarget. 2017. PMID: 29190894 Free PMC article.
-
Mimicking Retinoblastoma Treatment With Repeated Topotecan or Melphalan Develops Cross-Resistance to Classic Agents But Not to Repurposed Drugs.Invest Ophthalmol Vis Sci. 2024 Dec 2;65(14):14. doi: 10.1167/iovs.65.14.14. Invest Ophthalmol Vis Sci. 2024. PMID: 39636723 Free PMC article.
References
-
- Xu J, Liu Y, Yang Y, Bates S, Zhang JT. Characterization of oligomeric human half-ABC transporter ATP-binding cassette G2. J Biol Chem. 2004;279: 19781-19789. - PubMed
-
- Abbott BL. ABCG2 (BCRP) expression in normal and malignant hematopoietic cells. Hematol Oncol. 2003;21: 115-130. - PubMed
-
- Allen JD, Schinkel AH. Multidrug resistance and pharmacological protection mediated by the breast cancer resistance protein (BCRP/ABCG2). Mol Cancer Ther. 2002;1: 427-434. - PubMed
-
- Doyle LA, Ross DD. Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene. 2003;22: 7340-7358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

