GM-CSF regulates a PU.1-dependent transcriptional program determining the pulmonary response to LPS
- PMID: 16917076
- PMCID: PMC1899305
- DOI: 10.1165/rcmb.2006-0174OC
GM-CSF regulates a PU.1-dependent transcriptional program determining the pulmonary response to LPS
Abstract
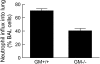
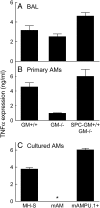
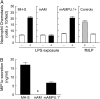
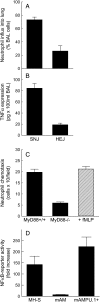
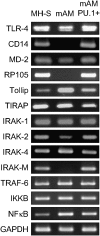
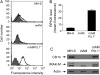
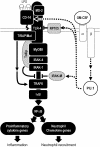
Alveolar macrophages (AMs) normally respond to lipopolysaccharide (LPS) by activating Toll-like receptor (TLR)-4 signaling, a mechanism critical to lung host defense against gram-negative bacteria such as Pseudomonas aeruginosa. Because granulocyte macrophage colony-stimulating factor (GM-CSF)-deficient (GM(-/-)) mice are hyporesponsive to LPS, we evaluated the role of GM-CSF in TLR-4 signaling in AMs. Pulmonary TNF-alpha levels and neutrophil recruitment 4 h after intratracheal administration of Pseudomonas LPS were reduced in GM(-/-) compared with wild-type (GM(+/+)) mice. Secretion of TNF-alpha by AMs exposed to LPS ex vivo was also reduced in GM(-/-) mice and restored in mice expressing GM-CSF specifically in the lungs (SPC-GM(+/+)/GM(-/-) mice). LPS-dependent NF-kappaB promoter activity, TNF-alpha secretion, and neutrophil chemokine release were reduced in AM cell lines derived from GM(-/-) mice (mAM) compared with GM(+/+) (MH-S). Retroviral expression of PU.1 in mAM cells, which normally lack PU.1, rescued all of these AM defects. To determine whether GM-CSF, via PU.1, regulated expression of TLR-4 pathway components, mRNA and protein levels for key components were evaluated in MH-S cells (GM(+/+), PU.1(Positive)), mAM cells (GM(-/-), PU.1(Negative)), and mAMPU.1+ cells (GM(-/-), PU.1(Positive)). Cluster of differentiation antigen-14, radioprotective 105, IL-1 receptor-associated kinase (IRAK)-M mRNA, and protein were dependent upon GM-CSF and restored by expression of PU.1. In contrast, expression of other TLR-4 pathway components (myeloid differentiation-2, TLR-4, IRAK-1, IRAK-2, Toll/IL-1 receptor domain containing adapter protein/MyD88 adaptor-like, myeloid differentiation primary-response protein 88, IRAK-4, TNF receptor-associated factor-6, NF-kappaB, inhibitor of NF-kappaB kinase) were not GM-CSF or PU.1-dependent. These results show that GM-CSF, via PU.1, enables AM responses to P. aeruginosa LPS by regulating expression of a specific subset of components of the TLR-4 signaling pathway.
Figures
References
-
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998;282:2085–2088. - PubMed
-
- Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA Jr. MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell 1998;2:253–258. - PubMed
-
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol 2004;4:499–511. - PubMed
-
- Kobayashi K, Hernandez LD, Galan JE, Janeway CA Jr, Medzhitov R, Flavell RA. IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 2002;110:191–202. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

