The vestibular-related frontal cortex and its role in smooth-pursuit eye movements and vestibular-pursuit interactions
- PMID: 16917164
- PMCID: PMC1761700
The vestibular-related frontal cortex and its role in smooth-pursuit eye movements and vestibular-pursuit interactions
Abstract
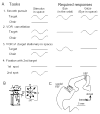
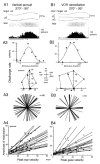
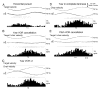
In order to see clearly when a target is moving slowly, primates with high acuity foveae use smooth-pursuit and vergence eye movements. The former rotates both eyes in the same direction to track target motion in frontal planes, while the latter rotates left and right eyes in opposite directions to track target motion in depth. Together, these two systems pursue targets precisely and maintain their images on the foveae of both eyes. During head movements, both systems must interact with the vestibular system to minimize slip of the retinal images. The primate frontal cortex contains two pursuit-related areas; the caudal part of the frontal eye fields (FEF) and supplementary eye fields (SEF). Evoked potential studies have demonstrated vestibular projections to both areas and pursuit neurons in both areas respond to vestibular stimulation. The majority of FEF pursuit neurons code parameters of pursuit such as pursuit and vergence eye velocity, gaze velocity, and retinal image motion for target velocity in frontal and depth planes. Moreover, vestibular inputs contribute to the predictive pursuit responses of FEF neurons. In contrast, the majority of SEF pursuit neurons do not code pursuit metrics and many SEF neurons are reported to be active in more complex tasks. These results suggest that FEF- and SEF-pursuit neurons are involved in different aspects of vestibular-pursuit interactions and that eye velocity coding of SEF pursuit neurons is specialized for the task condition.
Figures


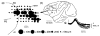




Similar articles
-
Role of vestibular signals in the caudal part of the frontal eye fields in pursuit eye movements in three-dimensional space.Ann N Y Acad Sci. 2005 Apr;1039:272-82. doi: 10.1196/annals.1325.026. Ann N Y Acad Sci. 2005. PMID: 15826981
-
Vestibular-related frontal cortical areas and their roles in smooth-pursuit eye movements: representation of neck velocity, neck-vestibular interactions, and memory-based smooth-pursuit.Front Neurol. 2011 Dec 14;2:78. doi: 10.3389/fneur.2011.00078. eCollection 2011. Front Neurol. 2011. PMID: 22174706 Free PMC article.
-
Latency of vestibular responses of pursuit neurons in the caudal frontal eye fields to whole body rotation.Exp Brain Res. 2007 Mar;177(3):400-10. doi: 10.1007/s00221-006-0682-5. Exp Brain Res. 2007. PMID: 16972072
-
Frontal cortical control of smooth-pursuit.Curr Opin Neurobiol. 2003 Dec;13(6):647-54. doi: 10.1016/j.conb.2003.10.007. Curr Opin Neurobiol. 2003. PMID: 14662364 Review.
-
The neural basis of smooth pursuit eye movements in the rhesus monkey brain.Brain Cogn. 2008 Dec;68(3):229-40. doi: 10.1016/j.bandc.2008.08.014. Epub 2008 Oct 2. Brain Cogn. 2008. PMID: 18835077 Review.
Cited by
-
Saccades to future ball location reveal memory-based prediction in a virtual-reality interception task.J Vis. 2013 Jan 16;13(1):20. doi: 10.1167/13.1.20. J Vis. 2013. PMID: 23325347 Free PMC article.
-
Otolith inputs to pursuit neurons in the frontal eye fields of alert monkeys.Exp Brain Res. 2009 Mar;193(3):455-66. doi: 10.1007/s00221-008-1644-x. Epub 2008 Nov 22. Exp Brain Res. 2009. PMID: 19030849
-
Gaze distribution analysis and saliency prediction across age groups.PLoS One. 2018 Feb 23;13(2):e0193149. doi: 10.1371/journal.pone.0193149. eCollection 2018. PLoS One. 2018. PMID: 29474378 Free PMC article. Clinical Trial.
-
An fMRI study on smooth pursuit and fixation suppression of the optokinetic reflex using similar visual stimulation.Exp Brain Res. 2008 Mar;185(4):535-44. doi: 10.1007/s00221-007-1176-9. Epub 2007 Oct 26. Exp Brain Res. 2008. PMID: 17962925
-
Activity of pursuit-related neurons in medial superior temporal area (MST) during static roll-tilt.Cereb Cortex. 2011 Jan;21(1):155-65. doi: 10.1093/cercor/bhq072. Epub 2010 Apr 26. Cereb Cortex. 2011. PMID: 20421248 Free PMC article.
References
-
- Akao T, Kurkin S, Fukushima K. Latency of adaptive vergence eye movements induced by vergence-vestibular interaction training in monkeys. Exp Brain Res. 2004;158:129–132. - PubMed
-
- Akao T, Kurkin S, Fukushima J, Fukushima K. Visual and vergence eye movement related responses of pursuit neurons in the caudal frontal eye fields to motion-in-depth stimuli. Exp Brain Res. 2005;164:92–108. - PubMed
-
- Akao T, Mustari MJ, Fukushima J, Kurkin S, Fukushima K. Discharge characteristics of pursuit neurons in MST during vergence eye movements. J Neurophysiol. 2005;93:2415–2434. - PubMed
-
- Akao T, Kasahara S, Kurkin S, Fukushima K. Coordinate frames in representing pursuit signals in simian frontal eye fields (FEF) J Physiol Sci. 2006;56(Suppl):S187. (Abstr).
-
- Amador N, Schlag-Rey M, Schlag J. Reward-predicting and reward-detecting neuronal activity in the primate supplementary eye field. J Neurophysiol. 2000;84:2166–2170. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
