Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein
- PMID: 16917503
- PMCID: PMC1560352
- DOI: 10.1038/sj.emboj.7601281
Identification of CD63 as a tissue inhibitor of metalloproteinase-1 interacting cell surface protein
Abstract
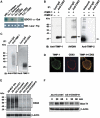
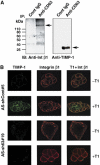
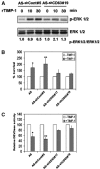
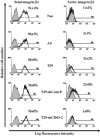
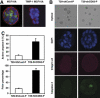
This study identified CD63, a member of the tetraspanin family, as a TIMP-1 interacting protein by yeast two-hybrid screening. Immunoprecipitation and confocal microscopic analysis confirmed CD63 interactions with TIMP-1, integrin beta1, and their co-localizations on the cell surface of human breast epithelial MCF10A cells. TIMP-1 expression correlated with the level of active integrin beta1 on the cell surface independent of cell adhesion. While MCF10A cells within a three-dimensional (3D) matrigel matrix form polarized acinar-like structures, TIMP-1 overexpression disrupted breast epithelial cell polarization and inhibited caspase-mediated apoptosis in centrally located cells, necessary for the formation and maintenance of the hollow acinar-like structures. Small hairpin RNA (shRNA)-mediated CD63 downregulation effectively reduced TIMP-1 binding to the cell surface, TIMP-1 co-localization with integrin beta1, and consequently reversed TIMP-1-mediated integrin beta1 activation, cell survival signaling and apoptosis inhibition. CD63 downregulation also restored polarization and apoptosis of TIMP-1 overexpressing MCF10A cells within a 3D-matrigel matrix. Taken together, the present study identified CD63 as a cell surface binding partner for TIMP-1, regulating cell survival and polarization via TIMP-1 modulation of tetraspanin/integrin signaling complex.
Figures
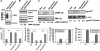
Similar articles
-
TIMP-1 modulates chemotaxis of human neural stem cells through CD63 and integrin signalling.Biochem J. 2014 May 1;459(3):565-76. doi: 10.1042/BJ20131119. Biochem J. 2014. PMID: 24635319
-
The tissue inhibitor of metalloproteinases-1 improves migration and adhesion of hematopoietic stem and progenitor cells.Exp Hematol. 2013 Sep;41(9):823-831.e2. doi: 10.1016/j.exphem.2013.04.010. Epub 2013 May 6. Exp Hematol. 2013. PMID: 23660069 Clinical Trial.
-
TIMP-1 via TWIST1 induces EMT phenotypes in human breast epithelial cells.Mol Cancer Res. 2014 Sep;12(9):1324-33. doi: 10.1158/1541-7786.MCR-14-0105. Epub 2014 Jun 3. Mol Cancer Res. 2014. PMID: 24895412 Free PMC article.
-
Characteristics of TIMP1, CD63, and β1-Integrin and the Functional Impact of Their Interaction in Cancer.Int J Mol Sci. 2021 Aug 27;22(17):9319. doi: 10.3390/ijms22179319. Int J Mol Sci. 2021. PMID: 34502227 Free PMC article. Review.
-
Novel functions of TIMPs in cell signaling.Cancer Metastasis Rev. 2006 Mar;25(1):99-113. doi: 10.1007/s10555-006-7893-x. Cancer Metastasis Rev. 2006. PMID: 16680576 Review.
Cited by
-
TIMP1 Indicates Poor Prognosis of Renal Cell Carcinoma and Accelerates Tumorigenesis via EMT Signaling Pathway.Front Genet. 2022 Feb 25;13:648134. doi: 10.3389/fgene.2022.648134. eCollection 2022. Front Genet. 2022. PMID: 35281807 Free PMC article.
-
Comparing TIMP-1 and Hsp70 in Blood and Saliva as Potential Prognostic Markers in HNSCC.Biomedicines. 2022 Dec 12;10(12):3225. doi: 10.3390/biomedicines10123225. Biomedicines. 2022. PMID: 36551979 Free PMC article.
-
Tissue Inhibitor of Metalloproteinase-1 Promotes Polymorphonuclear Neutrophil (PMN) Pericellular Proteolysis by Anchoring Matrix Metalloproteinase-8 and -9 to PMN Surfaces.J Immunol. 2019 Jun 1;202(11):3267-3281. doi: 10.4049/jimmunol.1801466. Epub 2019 Apr 24. J Immunol. 2019. PMID: 31019060 Free PMC article.
-
Low-density lipoprotein receptor-related protein-1 mediates endocytic clearance of tissue inhibitor of metalloproteinases-1 and promotes its cytokine-like activities.PLoS One. 2014 Jul 30;9(7):e103839. doi: 10.1371/journal.pone.0103839. eCollection 2014. PLoS One. 2014. PMID: 25075518 Free PMC article.
-
Tissue inhibitor of metalloproteinase-1 (TIMP-1) as a prognostic biomarker in gastrointestinal cancer: a meta-analysis.PeerJ. 2021 Feb 16;9:e10859. doi: 10.7717/peerj.10859. eCollection 2021. PeerJ. 2021. PMID: 33628641 Free PMC article.
References
-
- Avalos BR, Kaufman SE, Tomonaga M, Williams RE, Golde DW, Gasson JC (1988) K562 cells produce and respond to human erythroid-potentiating activity. Blood 71: 1720–1725 - PubMed
-
- Berditchevski F (2001) Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci 114: 4143–4151 - PubMed
-
- Berditchevski F, Bazzoni G, Hemler ME (1995) Specific association of CD63 with the VLA-3 and VLA-6 integrins. J Biol Chem 270: 17784–17790 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

