Roles of HIPK1 and HIPK2 in AML1- and p300-dependent transcription, hematopoiesis and blood vessel formation
- PMID: 16917507
- PMCID: PMC1560355
- DOI: 10.1038/sj.emboj.7601273
Roles of HIPK1 and HIPK2 in AML1- and p300-dependent transcription, hematopoiesis and blood vessel formation
Abstract
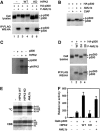
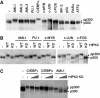
Histone acetyltransferases (HATs) p300 and CREB-binding protein (CBP) function as co-activators for a variety of sequence-specific transcription factors, including AML1. Here, we report that homeodomain-interacting protein kinase-2 (HIPK2) forms a complex with AML1 and p300, and phosphorylates both AML1 and p300 to stimulate transcription activation as well as HAT activities. Phosphorylation of p300 is triggered by phosphorylated AML1 as well as by PU.1, c-MYB, c-JUN and c-FOS, and is inhibited by dominant-negative HIPK2. Phosphorylation of p300 and AML1 is impaired in Hipk1/2 double-deficient mouse embryos. Double-deficient mice exhibit defects in primitive/definitive hematopoiesis, vasculogenesis, angiogenesis and neural tube closure. These phenotypes are in part similar to those observed in p300- and CBP-deficient mice. HIPK2 also phosphorylates another co-activator, MOZ, in an AML1-dependent manner. We discuss a possible mechanism by which transcription factors could regulate local histone acetylation and transcription of their target genes.
Figures







References
-
- Auerbach W, Dunmore JH, Fairchild-Huntress V, Fang Q, Auerbach AB, Huszar D, Joyner AL (2000) Establishment and chimera analysis of 129/SvEv- and C57BL/6-derived mouse embryonic stem cell lines. Biotechniques 29: 1024–1028, 1030, 1032 - PubMed
-
- Choi CY, Kim YH, Kim YO, Park SJ, Kim EA, Riemenschneider W, Gajewski K, Schulz RA, Kim Y (2005) Phosphorylation by the DHIPK2 protein kinase modulates the corepressor activity of Groucho. J Biol Chem 280: 21427–21436 - PubMed
-
- Choi CY, Kim YH, Kwon HJ, Kim Y (1999) The homeodomain protein NK-3 recruits Groucho and a histone deacetylase complex to repress transcription. J Biol Chem 274: 33194–33197 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

